Sperm and offspring production in a nonobstructive azoospermia mouse model via testicular mRNA delivery using lipid nanoparticles
- PMID: 41082659
- PMCID: PMC12557808
- DOI: 10.1073/pnas.2516573122
Sperm and offspring production in a nonobstructive azoospermia mouse model via testicular mRNA delivery using lipid nanoparticles
Abstract
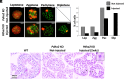
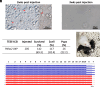
Microsurgical testicular sperm extraction (microTESE) with intracytoplasmic sperm injection (ICSI) represents the current standard treatment for nonobstructive azoospermia (NOA). However, cures remain unavailable for NOA patients lacking retrievable haploid cells. mRNA supplementation could be a potential treatment for genetic defects leading to impaired spermatogenesis. Lipid nanoparticles (LNPs) have emerged as mRNA delivery vehicles with minimal risk of genome integration; however, their ability to selectively deliver mRNA to specific cell types remains limited. To overcome this, microRNA (miRNA) target sequences were incorporated into mRNA constructs to restrict expression specifically to germ cells. Using pyruvate dehydrogenase E1 subunit alpha 2 (PDHA2) knockout mice as an NOA model with meiotic arrest, we demonstrate that LNP-mediated delivery of Pdha2 mRNA enables the resumption and completion of meiosis, restores sperm production, and facilitates the generation of healthy fertile offspring via ICSI. Whole-genome sequencing of the offspring confirmed the absence of large-scale genomic abnormalities. Our results provide proof of concept for a safe and effective chemically synthesized LNP-based mRNA therapy with miRNA-regulated germ cell specificity, offering a promising therapeutic approach to treating male infertility caused by spermatogenesis arrest.
Keywords: LNP therapy; azoospermia; germline therapy; lipid nanoparticle; sterility.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Farley T. M. M., “The WHO standardized investigation of the infertile couple” in Infertility Male and Female, vol. 4 Proceedings of the 12th World Congress on Fertility and Sterility Singapore, October 1986, Ratnam S. S., Reoh E.-S., Anandakumar C., Eds. (Parthenon Publishing Group, Camforth, Lanes., UK, 1987), pp 123–135.
-
- Gamidov S., et al. , Challenges in differential diagnosis between obstructive and non-obstructive azoospermia. UroPrecision 2, 30–35 (2024), 10.1002/uro2.45. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
- JP21H05033/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23jf0126001/Japan Agency for Medical Research and Development (AMED)
- JP23fa627006/Japan Agency for Medical Research and Development (AMED)
- JP23K20043/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01 HD088412/HD/NICHD NIH HHS/United States
- JPMJCR21N1/MEXT | Japan Science and Technology Agency (JST)
- JP22H04922/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP25H01353/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23fa627002/Japan Agency for Medical Research and Development (AMED)
- R01HD088412/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources

