Tepsin and AP4 mediate transport from the trans-Golgi to the plant-like vacuole in toxoplasma
- PMID: 41082686
- PMCID: PMC12517565
- DOI: 10.1083/jcb.202312109
Tepsin and AP4 mediate transport from the trans-Golgi to the plant-like vacuole in toxoplasma
Abstract
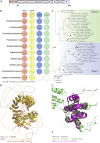
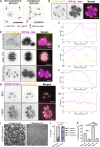
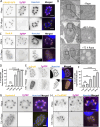
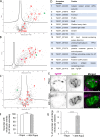
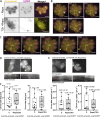
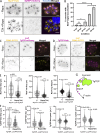
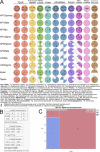
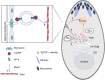
Apicomplexan parasites are obligate intracellular pathogens possessing unique organelles but lacking several components of the membrane trafficking machinery conserved in other eukaryotes. While some of these components have been lost during evolution, others remain undetectable by standard bioinformatics approaches. Using a conditional splitCas9 system in Toxoplasma gondii, we previously identified TGGT1_301410, a hypothetical gene conserved among apicomplexans, as a potential trafficking factor. Here, we show that TGGT1_301410 is a distant ortholog of T. gondii tepsin (TgTEP), localized to the trans-Golgi and functioning as an accessory protein of the adaptor protein complex 4 (AP4). We demonstrate that AP4-TgTEP is essential for the actin-dependent transport of vesicles to the plant-like vacuole (PLVAC) and Golgi organization. Notably, our findings reveal that, unlike in metazoans, the AP4 complex in T. gondii utilizes clathrin as a coat protein, a mechanism more closely aligned with that of plants. These results underscore a conserved yet functionally adapted vesicular transport system in Apicomplexa.
© 2025 Grech et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





References
-
- Alvarez-Jarreta, J., Amos B., Aurrecoechea C., Bah S., Barba M., Barreto A., Basenko E.Y., Belnap R., Blevins A., Böhme U., et al. 2024. VEuPathDB: The eukaryotic pathogen, vector and host bioinformatics resource center in 2023. Nucleic Acids Res. 52:D808–D816. 10.1093/nar/gkad1003 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

