ALKBH8-mediated codon-specific translation promotes colorectal tumorigenesis
- PMID: 41083459
- PMCID: PMC12518840
- DOI: 10.1038/s41467-025-64144-0
ALKBH8-mediated codon-specific translation promotes colorectal tumorigenesis
Abstract
Reprogramming gene expression at the translational level drives intestinal tumorigenesis. Codon decoding during translation elongation relies on tRNA modifications, while their pathological relevance in colorectal cancer remains to be elucidated. Here, we show that AlkB homolog 8 (ALKBH8), a uridine 34 (U34) tRNA methyltransferase, is a direct target of Wnt/β-catenin and is upregulated in colorectal cancer. Genetic ablation of ALKBH8 inhibits the development of intestinal tumors in Apcmin/+, azoxymethane/dextran sulfate sodium (AOM/DSS), and xenograft models. Loss of ALKBH8 induces ribosome pausing at adenine-ending codons, impairing the translation elongation of mRNAs enriched with these codons. Specifically, ALKBH8 regulates the translation of KRAS proto-oncogene in a codon-dependent manner. Rescue experiments demonstrate that the methyltransferase activity of ALKBH8 is required for its translation-promoting function. Together, our findings reveal ALKBH8-dependent mRNA translation as a critical mediator of intestinal tumorigenesis, underscoring its potential as a promising target for colorectal cancer therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

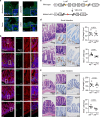
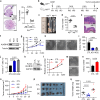


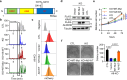

References
-
- Fearon, E. R. Molecular genetics of colorectal cancer. Annu Rev. Pathol.6, 479–507 (2011). - PubMed
-
- Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell61, 759–767 (1990). - PubMed
-
- Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell169, 985–999 (2017). - PubMed
-
- Morin, P. J., Kinzler, K. W. & Sparks, A. B. beta-catenin mutations: Insights into the APC Pathway and the power of genetics. Cancer Res76, 5587–5589 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

