Cerebrospinal fluid markers link to synaptic plasticity responses and Alzheimer's disease genetic pathways
- PMID: 41084010
- PMCID: PMC12519626
- DOI: 10.1186/s13024-025-00899-w
Cerebrospinal fluid markers link to synaptic plasticity responses and Alzheimer's disease genetic pathways
Abstract
Background: Synapse loss is linked to cognitive symptoms in Alzheimer's Disease (AD) and Cerebrospinal fluid (CSF) synaptic biomarkers may clarify disease heterogeneity and disease mechanisms for progression beyond amyloid (Aβ) and tau pathologies, potentially revealing new drug targets.
Methods: We used a mass-spectrometry panel of 17 synaptic biomarkers including neuronal pentraxins (NPTXs) linked to glutamatergic signaling, and 14-3-3 proteins linked to tau-pathology and synaptic plasticity. Synapse markers were evaluated in two independent cohorts: Dementia Disease Initiation (DDI) (n = 346) and Amsterdam Dementia Cohort (n = 397), both with cognitive assessments up to 10 years. We used linear regression to compare synapse marker differences between CSF-determined Aβ + cognitively normal (CN) and Mild Cognitive Impairment (MCI) groups, with or without CSF tau pathology (Tau+/-), relative to CN Aβ-/Tau- controls; and associations between synapse markers and medial temporal lobe (MTL) MRI volumetrics in the DDI cohort and with verbal memory in both cohorts. A funneling procedure identified proteins related to Aβ/Tau pathology and memory impairment in both cohorts, which were used to evaluate relations to Aβ/Tau biological progression in the DDI cohort and memory decline in both cohorts. Finally, we explored genetic pathways associated with these synaptic proteins.
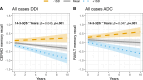
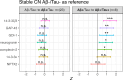
Results: In both cohorts, most markers were elevated in Aβ+/Tau + cases compared to controls, particularly 14-3-3ζ/δ. Several proteins were reduced in Aβ+/Tau- cases, especially NPTX-2, while 14-3-3ζ/δ remained elevated. However, the increase in e.g. 14-3-3ζ/δ and reduction in e.g. NPTX2 were more pronounced in patients with MCI than CN cases regardless of tau-pathology, corresponding to verbal memory impairment and MTL atrophy. Elevated baseline 14-3-3ζ/δ and rab GDP Dissociation Inhibitor Alpha (GDI-1) associated with future progression from Aβ+/Tau- to Aβ+/Tau+. Significant associations (all p < 0.001) were found between 14-3-3 protein genes (YWHAZ, YWHAE) and pathways linked to AD, including the p38 MAPK, IGF, PIK3/AKT and between GDI1 and p38 MAPK upstream pathway (p < 0.05) all connected to synaptic plasticity. Correspondingly, a robust 14-3-3ζ/δ association with future memory decline was observed in both cohorts.
Conclusions: Reduced markers for excitatory signaling in Aβ+/Tau- and increased synaptic plasticity markers in Aβ+/Tau + cases suggest differential but linked processes underlying disease progression and resilience in the groups.
Keywords: Alzheimer’s disease; Cognition; Memory; Neurodegeneration; Synaptic loss.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The DDI study was approved by the Regional Committees for Medical and Health Research Ethics in Norway and conducted in line with the guidelines provided by the Helsinki declaration, and the Norwegian Health and Research act. All participants volunteered and gave written informed consent before participating in the study. All patients in the ADC study gave written informed consent, was in line with the guidelines provided by the Helsinki declaration, was approved by the ethics committee of the Amsterdam UMC (location VUmc), the Biobank Research Ethics Committee of the Amsterdam UMC (location VUmc). Consent for publication: All authors have reviewed and approved the contents of this manuscript and have provided their consent for publication. Competing interests: BEK has served as a consultant for Biogen and on an advisory board for Eisai and Eli Lilly. TF has served as a consultant and at the advisory boards for Biogen, Novo Nordisk, Eli Lilly, Roche and Eisai. KB has served as a consultant and at advisory boards for Abbvie, AC Immune, ALZPath, AriBio, Beckman-Coulter, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Quanterix, Roche Diagnostics, Sanofi and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). PS has served as a consultant for Roche. RES has served on an advisory board for Eisai and as local PI on GSK 219867. DA has received research support and/or honoraria from, Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, Biogen, and GE Health, and served as paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura and Cognetivity. All other authors declare that they have no competing interests.
Figures




References
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi M. Forecasting the global burden of Alzheimer´s disease. Alzheimer´s Dement. 2007;3(3):186–91. - PubMed
-
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

