Transfusion of Amustaline/Glutathione Pathogen-reduced Red Blood Cells in Cardiac Surgery: A Randomized Phase 3 Clinical Trial
- PMID: 41085306
- PMCID: PMC12513042
- DOI: 10.1097/ALN.0000000000005716
Transfusion of Amustaline/Glutathione Pathogen-reduced Red Blood Cells in Cardiac Surgery: A Randomized Phase 3 Clinical Trial
Abstract
Background: Transfusion has a persistent low risk of transfusion-transmitted infection and transfusion-associated graft-versus-host disease that may be addressed using pathogen reduction. The Red Cell Pathogen Inactivation (ReCePI) trial tested whether amustaline/glutathione pathogen-reduced red cells are noninferior to conventional transfusions for support of acute surgical blood loss.
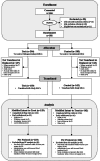
Methods: A phase 3, double-blinded, noninferiority trial randomized cardiac or thoracic-aorta surgery patients with increased risk of red cell transfusion to receive pathogen-reduced or conventional red cells during and for 7 days postsurgery. The primary endpoint was the proportion of patients with acute kidney injury (AKI), which is defined as an increase from baseline of greater than or equal to 0.3 mg/dl serum creatinine within 48 h of surgery. Noninferiority was claimed if the upper bound 95% CI of the treatment difference was less than half (50%) of the observed conventional arm incidence. Adverse events and treatment-emergent red cell antibodies were assessed for 28 and 75 days, respectively.
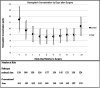
Results: A total of 581 subjects were randomized, and 321 (55%) were transfused with study red cells. Transfused subjects in both arms had similar baseline demographics, medical histories, hemoglobin levels, and surgical procedures. Hemoglobin day 3 nadir levels (8.6 g/dl [7.8 to 9.2] in the pathogen-reduced arm; 8.4 g/dl [7.8 to 9.3] in the conventional arm; P = 0.52) were comparable. Incidence of AKI by 48 h was 46 of 157 (29.3%) in the pathogen-reduced arm and 45 of 161 (28.0%) in the conventional arm (treatment difference, 0.7%; 95% CI, -8.9 to 10.4%; noninferiority margin, 14.0%; P = 0.001 for noninferiority). AKI within 7 days by Kidney Disease Improving Global Outcomes staging criteria was not different (59 of 159 [37.1%] in the pathogen-reduced arm; 55 of 162 [34.0%] in the conventional arm; P = 0.53), but stage III was more common in the pathogen-reduced arm (pathogen-reduced arm, 15 of 159 [9.4%]; conventional arm, 7 of 162 [4.3%]; P = 0.075). Of 159 pathogen-reduced red cell recipients, 5 (3.1%) developed specific, low-titer antibodies without evidence of hemolysis.
Conclusions: The incidence of AKI in recipients of pathogen-reduced red cells was noninferior to conventional red cell transfusion. Treatment-emergent antibodies were uncommon and not clinically significant.
Trial registration: ClinicalTrials.gov NCT03459287.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc., on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
Author Dr. Benjamin and collaborators Drs. Corash, Liu, Bentow, Mufti, Varrone, and Pitman are employees of Cerus Corporation (Concord, California), the sponsor of the study. Authors Drs. Snyder, Sekela, Welsby, Toyoda, Alsammak, Sodha, Beaver, Pelletier, Gorham, McNeil, Sniecinski, Pearl, Nuttall, Sarode, and Reece and collaborators Drs. Kaplan, Davenport, Ipe, Gammon, Sadler, and Lopez-Plaza are investigators on the study, receive no compensation from the sponsor, and declare no competing interests with this work. Author Dr. Welsby reports consulting fees from Pfizer Inc. (New York City, New York). Author Dr. Gorham reports travel reimbursement from the American Red Cross (Washington, D.C.). Author Dr. McNeil reports consulting fees from Medtronic (Minneapolis, Minnesota). Collaborator Dr. Benharash reports compensation from Atricure (Maon, Ohio) as a surgical proctor. Author Dr. Sniecinski reports consulting fees from Anesthesia Labs, Inc. (San Francisco, California) and is an officer of Tractio Diagnostics, Inc. (Camden, Deleware). Author Dr. Sarode reports consulting fees from Octapharma (Paramus, New Jersey), VARMX (Leiden, Netherlands), and Prothya Biosolutions BV (Bruseels). The other authors declare no competing interests.
Figures


References
-
- Kopolovic I, Ostro J, Tsubota H, et al. : A systematic review of transfusion-associated graft-versus-host disease. Blood 2015; 126:406–14. doi:10.1182/blood-2015-01-620872 - PubMed
-
- Matos D, Tomashek KM, Perez-Padilla J, et al. : Probable and possible transfusion-transmitted dengue associated with NS1 antigen-negative but RNA confirmed-positive red blood cells. Transfusion 2016; 56:215–22. doi:10.1111/trf.13288 - PubMed
-
- Snyder EL, Stramer SL, Benjamin RJ: The safety of the blood supply—Time to raise the bar. N Engl J Med 2015; 373:882. doi:10.1056/NEJMc1507761 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

