Region-specific drivers of CSF mobility measured with MRI in humans
- PMID: 41087750
- PMCID: PMC12586159
- DOI: 10.1038/s41593-025-02073-3
Region-specific drivers of CSF mobility measured with MRI in humans
Abstract
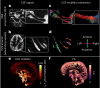
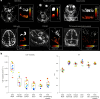
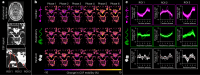
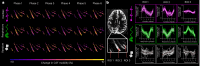
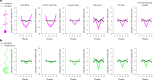
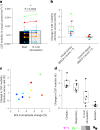
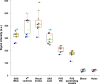
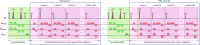
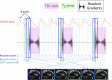
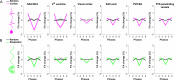
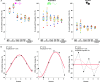
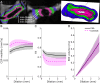
Many neurological diseases are characterized by the accumulation of toxic proteins in the brain. This accumulation has been associated with improper clearance from the parenchyma. Recent discoveries highlighted perivascular spaces, which are cerebrospinal fluid (CSF)-filled spaces, as the channels of brain clearance. The forces driving CSF mobility within perivascular spaces are still debated. Here we present a noninvasive, CSF-specific magnetic resonance imaging technique (CSF-Selective T2-prepared REadout with Acceleration and Mobility-encoding) that enables detailed in vivo measurement of CSF mobility in humans, down to the level of perivascular spaces located around penetrating vessels, which is close to protein production sites. We find region-specific drivers of CSF mobility and demonstrate that CSF mobility can be increased by entraining vasomotion. Furthermore, we find region-specific CSF mobility alterations in patients with cerebral amyloid angiopathy, a brain disorder associated with clearance impairment. The availability of this technique opens up avenues to investigate the impact of CSF-mediated clearance in neurodegeneration and sleep.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The LUMC receives research support from Philips. M.W.A.C. is a shareholder of Nicolab International Ltd. K.D. and D.P. are co-founders and shareholders of relios.vision GmbH. D.P. is on the Guerbet advisory board. The other authors declare no competing interests.
Figures


References
-
- Dolgin, E. Brain’s drain. Nat. Biotechnol.38, 258–262 (2020). - PubMed
MeSH terms
Grants and funding
- WE.25-2020-05/Alzheimer Nederland (Alzheimer Netherlands)
- 23CVD03/Fondation Leducq
- 2022_EKCS.17/Else Kröner-Fresenius-Stiftung (Else Kroner-Fresenius Foundation)
- 2022-EKES.33/Else Kröner-Fresenius-Stiftung (Else Kroner-Fresenius Foundation)
- 01KX2130/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources
Medical

