POST introduction evaluation (PIE) of the malaria vaccine introduced in three pilot countries (Ghana, Kenya, and Malawi) in 2021
- PMID: 41088219
- PMCID: PMC12522374
- DOI: 10.1186/s12936-025-05590-5
POST introduction evaluation (PIE) of the malaria vaccine introduced in three pilot countries (Ghana, Kenya, and Malawi) in 2021
Abstract
Background: The World Health Organization (WHO) recommends the use of malaria vaccines for the prevention of Plasmodium falciparum malaria in moderate to high transmission areas, administered in a 4-dose schedule in children from 5 months of age. The vaccine is a ground-breaking new tool to add to the existing package of recommended malaria interventions to reduce malaria morbidity and mortality. Ghana, Kenya, and Malawi were the first countries to introduce the RTS,S/AS01E (RTS,S) malaria vaccine into their childhood immunization programmes in 2019 as part of a pilot programme called the Malaria Vaccine Implementation Programme (MVIP).
Methods: The WHO's post-introduction evaluation (PIE) methodology was adapted to evaluate malaria vaccine implementation in each of the three pilot countries at least a year after the vaccine's introduction. Semi-structured questionnaires were used to interview immunization staff at national, sub-national, and health facility levels, supplemented with systematic observations of vaccination sessions and vaccine storage sites. At the health facility, a sample of caregivers of eligible children was also interviewed. Sites were purposively selected to include a range of past immunization coverage and varied demographics among the populations served.
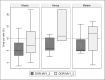
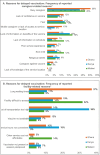
Results: All three countries successfully introduced the malaria vaccine during the MVIP. Reported malaria vaccine median coverage at least 2 years after the start of the pilot ranged from 69-91% for dose 1, 62-82% for dose 2, to 58-81% for dose 3 by 24-30 months from the start of the pilot. Coverage for dose 4 was lower as fewer children were eligible during the PIE reporting timeframe. Best practices identified during the PIEs included: early involvement of subnational stakeholders; advance updating and distribution of recording and reporting tools to include malaria vaccine; pre-assessment of cold chain capacity and scale-up; investment of time and resources in health worker trainings and refreshers; involvement of community health workers; robust defaulter tracing mechanisms; ensuring community "dialogue" with continuity of advocacy, communication, and social mobilization activities after initial introduction; regular onsite supervisory visits before, during and after introduction; and use of social media for messaging.
Conclusions: Malaria vaccine is an important intervention as part of a comprehensive malaria control strategy. Conducting a PIE is useful to identify best practices and lessons learned. New vaccination contacts take time to establish and achieve high coverage as communities become aware of and understand when, why, and how to access the malaria vaccine. The malaria vaccine was successfully introduced as part of the routine childhood immunization programme with strong intersectoral collaboration and planning, involving both immunization and malaria stakeholders, comprehensive training, and social mobilization efforts pre- and post-introduction.
Keywords: Ghana; Kenya; Malaria vaccine; Malaria vaccine implementation programme; Malaria vaccine pilot introduction; Malawi]; New vaccine introduction; Post-introduction evaluation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Disclaimer: The findings and conclusions expressed in this report do not necessarily represent the decisions, policies, or views of the WHO. Competing interests: The authors declare no competing interests.
Figures

References
-
- WHO. World Malaria Report 2024. Geneva, World Health Organization; 2024.
-
- WHO. Malaria vaccine implementation programme. Geneva, World Health Organization. [Available from: https://www.who.int/initiatives/malaria-vaccine-implementation-programme.
-
- WHO. New vaccines post-introduction evaluation (PIE) tool. Geneva, World Health Organization; 2010.
-
- Asante KP, Mathanga DP, Milligan P, Akech S, Oduro A, Mwapasa V, et al. Feasibility, safety, and impact of the RTS, S/AS01E malaria vaccine when implemented through national immunisation programmes: evaluation of cluster-randomised introduction of the vaccine in Ghana, Kenya, and Malawi. Lancet. 2024;403:1660–70. - PMC - PubMed
-
- WHO. Malaria vaccines WHO position paper. Wkly Epidemiol Rec. 2024;99:225–48.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

