Bridging the Diagnostic Gap: A Rapid, Cost-Effective, and Equitable Hepatitis C Virus RNA Detection Method for Resource-Limited Settings
- PMID: 41089093
- PMCID: PMC12516101
- DOI: 10.7759/cureus.92101
Bridging the Diagnostic Gap: A Rapid, Cost-Effective, and Equitable Hepatitis C Virus RNA Detection Method for Resource-Limited Settings
Abstract
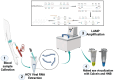
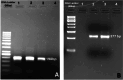
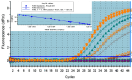
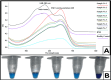
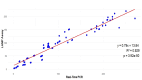
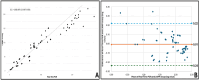
Hepatitis C virus (HCV) remains a major global health challenge, particularly in resource-limited settings where access to molecular diagnostics is restricted. The loop-mediated isothermal amplification (LAMP) assay offers a rapid, cost-effective alternative to polymerase chain reaction (PCR) for HCV RNA detection. Unlike PCR, LAMP uses isothermal amplification (60-65°C), eliminating the need for expensive thermal cyclers and yielding results in under 60 minutes. We evaluated two LAMP detection methods - hydroxynaphthol blue (HNB, colorimetric) and calcein (fluorescent) - against an in-house TaqMan qPCR assay. Both LAMP methods demonstrated high sensitivity (99.6%) and specificity (95.6% for HNB, 99.2% for calcein), with a broad dynamic range (10-10⁶ copies/mL). Clinical validation against real-time PCR showed strong agreement, with a positive predictive value of 98.6% and a negative predictive value of 94.4%. The HNB-LAMP assay, in particular, provides a simple visual readout (blue to sky blue), requiring no specialized equipment, while calcein-LAMP offers fluorescence-based detection under UV light. Both methods showed no significant correlation (p > 0.5), confirming their reliability. LAMP's advantages - minimal infrastructure, ambient temperature stability, and low cost (<$5 per test) - make it ideal for decentralized testing in low-resource settings. This approach could revolutionize HCV diagnosis by enabling same-day test-and-treat strategies, improving linkage to care, and supporting global HCV elimination efforts. Future steps include field validation in remote clinics, manufacturing scale-up, and integration into point-of-care platforms to maximize accessibility. By bridging the diagnostic gap, LAMP can potentially transform HCV management in underserved regions worldwide.
Keywords: bland-altman plot; calcein; hcv rna; hnb; icc; in-house qpcr; lamp methods.
Copyright © 2025, Prakash et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Institute's Ethical Committee of the All India Institute of Medical Sciences, New Delhi, issued approval IEC/NP-400/2013. Ethical clearance was obtained from the Institute's Ethical Committee of the All India Institute of Medical Sciences, New Delhi (Study code No # IEC/NP-400/2013). Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: This project was funded by the Department of Biotechnology, New Delhi (project code: N1465), and no funds are currently available. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Hepatitis C virus particle detected by immunoelectron microscopic study. Kaito M, Watanabe S, Tsukiyama-Kohara K, et al. J Gen Virol. 1994;75:1755–1760. - PubMed
-
- Structural biology of hepatitis C virus. Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Hepatology. 2004;39:5–19. - PubMed
-
- Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. https://pubmed.ncbi.nlm.nih.gov/27678366/ World J Gastroenterol. 2016;22:7824–7840. - PMC - PubMed
-
- Hepatitis C virus-induced steatosis: an overview. Negro F. Dig Dis. 2010;28:294–299. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
