The role of extracellular vesicles in murine asthma model: insights into IgE-independent mast cell activation within animal science
- PMID: 41089358
- PMCID: PMC12516586
- DOI: 10.5187/jast.2024.e65
The role of extracellular vesicles in murine asthma model: insights into IgE-independent mast cell activation within animal science
Abstract
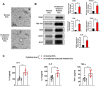
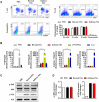
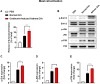
Asthma, a prevalent respiratory condition in animal science, involves bronchial inflammation, leading to symptoms like coughing and difficulty breathing. Mast cells and macrophages, pivotal immune cells, contribute to the inflammatory response triggered by various factors. Extracellular vesicles (EVs), including exosomes, play crucial roles in intercellular communication and have been implicated in murine asthma. We hypothesize that murine asthma-derived EVs modulate immune cell responses in animals. Our study investigates the role of these EVs in immune cell activation, shedding light on asthma pathogenesis. Using a murine asthma model induced by ovalbumin (OVA), we assessed serum EVs via Nanoparticle Tracking Analysis (NTA). Serum EVs from normal and asthmatic mice were subjected to Enzyme-linked immunosorbent assay (ELISA) and proteomic analysis for component identification. Elevated EV concentration and inflammatory cytokines, such as Interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α, were found in asthma-derived EVs. Additionally, variations in immunoglobulin light chain types were identified. Surprisingly, murine asthma EVs failed to activate T lymphocytes, B lymphocytes, eosinophils, and macrophages but stimulated mouse bone marrow-derived mast cells (mBMMCs) via enhanced degranulation and MAP kinase phosphorylation. These results suggest murine asthma-derived EVs as potential therapeutic targets for managing asthmatic symptoms in animal science. Further research into their mechanisms and clinical applications is warranted.
Keywords: Asthma; Extracellular vesicles; MAP kinase phosphorylation; Mast cells.
© Copyright 2025 Korean Society of Animal Science and Technology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Holgate ST. Asthma: a dynamic disease of inflammation and repair. In: Chadwick DJ, Cardew G, editors. Ciba Foundation symposium 206: the rising trends in asthma. Chicheste, West Susse: John Wiley & Sons; 1997. - PubMed
-
- Jung SH, Bae CH, Kim JH, Park SD, Shim JJ, Lee JL. Lactobacillus casei HY2782 and Pueraria lobata root extract complex ameliorates particulate matter-induced airway inflammation in mice by inhibiting Th2 and Th17 immune responses. Prev Nutr Food Sci. 2022;27:188–97. doi: 10.3746/pnf.2022.27.2.188. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

