Budget Impact of Adopting Nirmatrelvir-Ritonavir for Treating COVID-19 in a Large Integrated Healthcare System
- PMID: 41089767
- PMCID: PMC12517272
- DOI: 10.1093/ofid/ofaf596
Budget Impact of Adopting Nirmatrelvir-Ritonavir for Treating COVID-19 in a Large Integrated Healthcare System
Abstract
Background: Understanding the budget impact of prescribing nirmatrelvir-ritonavir (NR) for COVID-19 can inform procurement and allocation strategies in large healthcare systems.
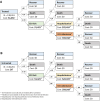
Methods: We assessed the budget impact of providing NR in the Veterans Health Administration (VHA) for all treatment-eligible Veterans (laboratory-confirmed COVID-19 illness from April 2022 through March 2023) and by clinical subgroups, including predicted hospitalization/death risk quartile. We used decision tree models that included 30-day emergency department (ED) visits, hospitalizations, and death to assess the budget impact of NR. Transition probabilities were derived from a target trial emulation of NR effectiveness in the same population. We priced NR at $1031/course and used cost accounting records to estimate ED ($1420), hospitalization ($22 419), and hospitalization with ICU ($59 918) costs.
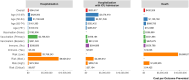
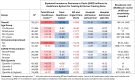
Results: Among 138 261 treatment-eligible Veterans, 18% (n = 24 892) were prescribed NR. Treating all patients compared with treating none reduced healthcare costs by -$20 million (uncertainty bound [UB]: -70-0.23) but increased total budget costs by +$122 million (UB: 73-143) due to NR purchasing costs. Targeted treatment of patients in the highest risk quartile (n = 19 406) achieved healthcare cost savings of -$17 million (UB: -49 to -3) and a modest total budget increase (+$3 million, UB: -29-17).
Conclusions: NR may reduce 30-day COVID-19 healthcare utilization costs, but the high cost of purchasing NR is likely to exceed those savings. Price reductions are necessary for NR to be a financially viable treatment for healthcare systems. Risk-informed allocation strategies can help maximize treatment benefits and minimize budget increases.
Keywords: COVID-19; antiviral; budget impact; cost; decision tree.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2025.
Conflict of interest statement
Potential conflicts of interest: None.
Figures

References
-
- U.S. Food & Drug Administration . FDA approves first oral antiviral for treatment of COVID-19 in adults. 9 August 2024. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-o.... Accessed 13 February 2025.
LinkOut - more resources
Full Text Sources
Miscellaneous

