Formation of an auditory sensory representation in posterior striatum emerges during a brief temporal window of associative learning in normal and hearing-impaired gerbils
- PMID: 41090114
- PMCID: PMC12515963
- DOI: 10.3389/fnsys.2025.1642595
Formation of an auditory sensory representation in posterior striatum emerges during a brief temporal window of associative learning in normal and hearing-impaired gerbils
Abstract
Introduction: The posterior tail of the striatum receives dense inputs from sensory regions of cortex and thalamus, as well as midbrain dopaminergic innervation, providing a neural substrate for associative sensory learning. Previously, we have demonstrated that developmental hearing loss is associated with aberrant physiological states in striatal medium spiny neurons (MSNs).
Methods: Here we directly investigated auditory associative learning impairments in the striatum of adult Mongolian gerbils that underwent transient developmental hearing loss or sham hearing loss during the critical period of auditory development. We used electrophysiology to reveal significant changes to neuronal population responses in vivo and intrinsic and synaptic properties to medium spiny neurons in vitro as animals learned an appetitive "Go/No-Go" auditory discrimination task. For in vivo experiments a 64-channel electrode was implanted in the auditory region of the posterior tail of the striatum and neuronal recordings were carried out as animals learned the task. For in vitro experiments, corticostriatal slice preparations were made from animals on each day of training.
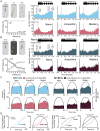
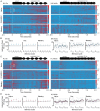
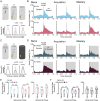
Results: In naïve animals from both groups there was limited to no phase locking to either auditory stimulus in vivo, and long term depression resulted from theta burst stimulation in vitro. Furthermore, intrinsic and synaptic properties in normal hearing animals were unaffected; however, the hearing loss group continued to show lowered synaptic inhibition, synaptic hyperexcitation, and suppressed intrinsic excitability in the hearing loss group. Starting around day 3-4 in both groups, the emergence of striatal medium spiny neuron phase locking to the auditory conditioning stimuli was observed in vivo. This occurred contemporaneous to an increased probability of theta burst induced LTP during MSN whole cell recording in vitro, and acquisition of the task as the correct rejection response significantly increased in the behaving animals. During the acquisition phase MSNs in the normal hearing group showed a significant decrease in synaptic inhibition and increase in synaptic excitation with no change to intrinsic excitability, while the MSNs in the hearing loss group showed a significant increase in synaptic inhibition, reduction of synaptic hyper excitability, and compensatory changes to intrinsic excitability that supported normal action potential generation. In both groups, synaptic properties were resolved to similar level of E/I balance that could be part of a conserved learning state.
Discussion: These changes to the intrinsic and synaptic properties likely support LTP induction in vivo and the strengthening of synapses between auditory inputs and MSNs that facilitate neuronal phase locking. These findings have significant implications for our understanding of striatal resilience to sensory impairments in early life, in addition to establishing a granular understanding of the striatal circuit changes that support reward driven stimulus-response learning.
Keywords: associative learning; auditory striatum; awake behaving recording; in vitro whole cell recording; in vivo electrophysiology; posterior striatum; tail striatum.
Copyright © 2025 Smith, Hong, Murphy, Chandrasekar, Dangcil, Nacipucha, Tucker, Carayannopoulos, Carayannopoulos, Peci, Kiel, Suresh, Guirguis, Utku, Paraouty, Gay, Wackym, Yao and Mowery.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

