Crystallization-Induced Diastereomer Transformations of Donor-Acceptor Cyclopropanes
- PMID: 41090293
- PMCID: PMC12571385
- DOI: 10.1021/jacs.5c14503
Crystallization-Induced Diastereomer Transformations of Donor-Acceptor Cyclopropanes
Abstract
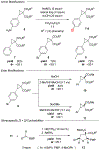
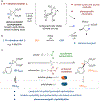
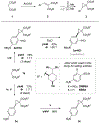
We report the first crystallization-induced diastereomer transformations (CIDTs) of donor-acceptor (D-A) cyclopropanes, providing access to important chiral nonracemic building blocks for stereospecific and stereoselective transformations. Lewis acids rapidly epimerize aniline-substituted D-A cyclopropanes through reversible C-C bond cleavage. Formation of the conjugate acid anilinium salt concurrently slows epimerization and enhances the crystallinity of the cyclopropanes. We present the first example of a temperature-dependent switchable stereoselective crystallization, which enables isolation of either diastereomer with high yield and diastereoenrichment. As a complement to the Lewis acid activation paradigm, solvent-promoted epimerization is demonstrated to enable a spontaneous CIDT of an aniline-substituted D-A cyclopropane in the absence of a Lewis acid. In both approaches, products can be isolated by direct filtration of the reaction mixture without the need for further purification. The latter approach was demonstrated on a multidecagram scale. Through manipulation of the aniline and ester functional handles, this method enables access to diastereo- and enantiopure cyclopropanes and derivatives thereof.
Figures


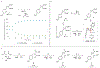

References
-
- Kolarovič A; Jakubec P State of the Art in Crystallization-Induced Diastereomer Transformations. Adv. Synth. Catal 2021, 363 (17), 4110–4158.
-
- Brands KMJ; Davies AJ Crystallization-Induced Diastereomer Transformations. Chem. Rev 2006, 106 (7), 2711–2733. - PubMed
-
- Noyori R; Ikeda T; Ohkuma T; Widhalm M; Kitamura M; Takaya H; Akutagawa S; Sayo N; Saito T; Taketomi T; Kumobayashi H Stereoselective Hydrogenation via Dynamic Kinetic Resolution. J. Am. Chem. Soc 1989, 111 (25), 9134–9135.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

