Glucose-Lowering Medication Classes and Cardiovascular Outcomes in Patients With Type 2 Diabetes
- PMID: 41091469
- PMCID: PMC12529185
- DOI: 10.1001/jamanetworkopen.2025.36100
Glucose-Lowering Medication Classes and Cardiovascular Outcomes in Patients With Type 2 Diabetes
Abstract
Importance: Major adverse cardiovascular events (MACEs) are primary causes of morbidity and mortality in adults with type 2 diabetes (T2D), yet few head-to-head randomized trials have compared the effects of glucose-lowering medications on MACEs, and most observational analyses are limited by inadequate bias adjustment methods.
Objective: To compare the effectiveness of sustained exposure to 4 classes of glucose-lowering medications (sulfonylureas, dipeptidyl peptidase-4 inhibitors [DPP4is], sodium-glucose cotransporter-2 inhibitors [SGLT2is], and glucagon-like peptide-1 receptor agonists [GLP-1RAs]) on MACEs in US adults with T2D using modern causal methods combined with machine learning.
Design, setting, and participants: This comparative effectiveness study included adults with T2D who were members of 6 large US health care delivery systems and initiated treatment with 1 of 4 medication classes (sulfonylureas, DPP4is, SGLT2is, and GLP-1RAs) between January 1, 2014, and December 31, 2021. Data analysis was conducted from May 1 to December 31, 2024.
Exposure: New use of a sulfonylurea, DPP4i, SGLT2i, or GLP-1RA based on filled prescriptions.
Main outcomes and measures: The primary outcome was MACEs defined as nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. Analyses were conducted using targeted learning within a trial emulation framework. Heterogeneity of treatment effects was assessed for prespecified subgroups.
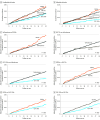
Results: This study included 296 676 adults. The cohort for emulating a 4-arm trial included a subset of 241 981 adults (mean [SD] age, 57.2 [12.9] years; 54.3% male) with T2D. In adjusted analyses, 2.5-year MACE risk was lowest in patients with sustained exposure to GLP-1RAs, followed by SGLT2is , sulfonylureas, and DPP4is. Comparing DPP4is with sulfonylureas and SGLT2is with GLP-1RAs, the 2.5-year cumulative risk difference was 1.9% (95% CI, 1.1%-2.7%) and 1.5% (1.1%-1.9%), respectively. Risk differences in patients with vs without atherosclerotic cardiovascular disease (ASCVD) were similar in direction but typically much smaller for patients without ASCVD. Evidence of a benefit of GLP-1RAs over SGLT2is was most pronounced in patients with baseline ASCVD or heart failure (HF), age 65 years or older, or low to moderate kidney impairment but was not found in patients younger than 50 years.
Conclusions and relevance: In this study, MACE risk varied significantly by medication class, with most protection achieved with sustained treatment with GLP-1RAs followed by SGLT2is, sulfonylureas, and DPP4is. The magnitude of benefit of GLP-1RAs over SGLT2is depended on baseline age, ASCVD, HF, and kidney impairment. These results, along with consideration of cost, availability, and collateral clinical benefits, may inform treatment decisions for adults with T2D.
Conflict of interest statement
Figures

References
-
- US Department of Health and Human Services (DHHS), Food and Drug Administration Center for Drug Evaluation and Research . Guidance for industry on diabetes mellitus: evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Fed Regist. 2008;73(245):77724-77725.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

