Key outcomes in treatment of activated phosphoinositide 3-kinase delta syndrome: An e-Delphi panel study and responder threshold application
- PMID: 41091701
- PMCID: PMC12527126
- DOI: 10.1371/journal.pone.0333341
Key outcomes in treatment of activated phosphoinositide 3-kinase delta syndrome: An e-Delphi panel study and responder threshold application
Abstract
Background: Activated phosphoinositide 3-kinase delta syndrome (APDS) is an ultra-rare, underrecognized inborn error of immunity. This study aimed to identify outcomes important in evaluating APDS treatment effectiveness and percent change in specific outcomes indicating a clinically meaningful benefit.
Methods: In this e-Delphi panel study, 28 globally based APDS experts used a 5-point Likert scale (Strongly Disagree to Strongly Agree) to indicate level of agreement that an outcome was an important measure of APDS treatment effectiveness in adult and pediatric patients at 3 and 6 months after treatment initiation. A threshold of ≥75% responding with "Agree" or "Strongly Agree" was considered consensus. Percent meaningful improvement in 6 outcomes was assessed and applied to APDS trial data (NCT02435173).
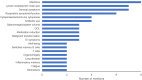
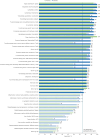
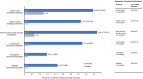
Results: Twenty-four panelists participated; e-Delphi rounds 1-5 were completed by 23, 21, 18, 17, and 16 panelists, respectively. Outcomes with the highest degree of consensus included lymph node size/volume, clinician overall impression of disease activity, antibiotic use, patient/caregiver-reported social outcomes and patient quality of life, hospitalizations, thrombocytopenia, spleen volume, lymphopenia, and anemia. Panelists indicated within-patient clinically meaningful improvements in adult patients ranged from median values of 20%-25% in lymph nodes, naïve B-cell to total B-cell ratio, spleen volume, hemoglobin, platelets, and lymphocytes at 3 months, and 25%-30% at 6 months. Panelists indicated within-patient clinically meaningful improvements in pediatric patients ranged from median values of 20%-27.5% at 3 months and 22.5%-45% at 6 months in the same 6 outcomes. In an application of responder thresholds, treatment with leniolisib resulted in significant and meaningful improvements in disease hallmarks, including lymph node size, spleen volume, and naïve B-cell ratio.
Conclusion: This study provides expert consensus on outcomes important in assessing APDS treatment effectiveness and improvement thresholds in 6 treatment outcomes indicative of a clinically meaningful benefit. These outcomes may help optimize APDS treatment in the clinic.
Copyright: © 2025 Upton et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: AC, AS, and JBeC received consulting fees from Pharming Healthcare, Inc. JEMU and KWW have received consulting fees and are steering committee members of Pharming Healthcare, Inc. JEMU has received advising fees from Pfizer and Bausch Health, speaker fees from AstraZeneca, clinical trial funding (to institution) from Regeneron and Sanofi, and drug donation for clinical trial use from Novartis. CG receives consulting fees from Pharming Healthcare, Inc. AH and JB are employees of Pharming Healthcare, Inc.
Figures




References
-
- Population Reference Bureau. 2023 World population data sheet. Accessed August 6, 2024. https://2023-wpds.prb.org/
-
- Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2022;42(7):1473–507. doi: 10.1007/s10875-022-01289-3 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

