Long-term comparative effectiveness of once-weekly semaglutide versus alternative treatments in a real-world US adult population with type 2 diabetes: a randomized pragmatic clinical trial
- PMID: 41093600
- PMCID: PMC12530410
- DOI: 10.1136/bmjdrc-2025-005161
Long-term comparative effectiveness of once-weekly semaglutide versus alternative treatments in a real-world US adult population with type 2 diabetes: a randomized pragmatic clinical trial
Abstract
Introduction: This study evaluated the long-term effectiveness of once-weekly subcutaneous semaglutide versus alternative treatment in adults with type 2 diabetes (T2D) in routine clinical practice.
Research design and methods: The SEmaglutide PRAgmatic (SEPRA) was a 2-year, randomized, open-label, pragmatic clinical trial (NCT03596450). Adults with T2D and inadequate glycemic control on one or two oral antidiabetic medications were randomized to receive once-weekly subcutaneous semaglutide or alternative treatment (chosen by the treating physician) as add-on therapy. Endpoints included proportion of participants achieving glycated hemoglobin (HbA1c)<7.0% at year 1 (primary endpoint) and year 2; changes in HbA1c (percentage point), body weight, and patient-reported outcomes (PROs) at years 1 and 2; and treatment changes (baseline to year 2). Missing data were imputed for some analyses.
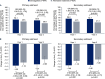
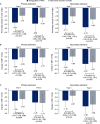
Results: Participants were randomized to semaglutide (n=644) or alternative treatment (n=634). Proportions of participants achieving HbA1c <7.0% were significantly higher with semaglutide versus alternative treatment at years 1 (53.1% vs 45.5%; OR (95% CI): 1.36 (1.03 to 1.79); p=0.033) and 2 (49.9% vs 38.9%; OR (95% CI): 1.56 (1.13 to 2.16); p=0.007). Mean HbA1c decreases were larger with semaglutide versus alternative treatment at year 1 (-1.35% vs -1.16%; estimated treatment difference (ETD) (95% CI): -0.20% (-0.39% to 0.00%); p=0.046) and year 2 (-1.27% vs -0.96%; ETD (95% CI): -0.31% (-0.57% to -0.05%); p=0.018). Semaglutide was associated with larger reductions in body weight at year 1 (-3.57% vs -1.91%; ETD (95% CI): -1.65% (-2.92% to -0.39%); p=0.010) but not year 2 (p=0.175). Treatment changes occurred less frequently with semaglutide than with alternative treatments. Some PROs indicated greater improvement with semaglutide versus alternative treatment. No new safety concerns were identified.
Conclusions: SEPRA demonstrates that semaglutide is an appropriate choice for treatment intensification among individuals with T2D in the USA who are receiving 1-2 antidiabetic medications. Findings support semaglutide as an effective and well-tolerated option in clinical practice.
Trial registration number: NCT03596450.
Keywords: Diabetes Mellitus, Type 2; Glucagon-Like Peptide 1.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JBB reports research support from Corcept, Dexcom, and Novo Nordisk; consulting fees from Altimmune, Antag, Amgen, ApStem, Aqua Medical, AstraZeneca, Boehringer-Ingelheim, CeQur, Corcept Therapeutics, Dexcom, Eli Lilly, Embecta, GentiBio, Glyscend, Insulet, Medtronic MiniMed, Mellitus Health, Metsera, Novo Nordisk, Pendulum Therapeutics, Praetego, Stability Health, Tandem, Terns, Vertex, and Zealand Pharma; and stock options from Glyscend, Mellitus Health, Metsera, Pendulum Therapeutics, Praetego, and Stability Health. HNC received support for the present manuscript from Novo Nordisk as an employee and holds stock or stock options for Novo Nordisk A/S. BJH received support for the present manuscript from Novo Nordisk as sponsor of the SEPRA trial; and contracted with his employer, Carelon Research, to serve as the data coordinating center for the trial, performing all data collection and analysis and supporting manuscript writing activities. MJC received support for the present manuscript from his employer, Carelon Research, which received funding from Novo Nordisk to perform the study services. He also holds stock or stock options for Elevance Health as a stockholder in ELV, which is the parent company of Carelon Research. VJW received support for the present manuscript from his employer, Carelon Research, which received funding from Novo Nordisk to perform the study services. He also holds stock or stock options for Elevance Health as a stockholder in ELV, which is the parent company of Carelon Research. SS received support for the present manuscript from Novo Nordisk as a full-time employee and holds stock or stock options for Novo Nordisk.
Figures


References
-
- International Diabetes Federation IDF diabetes atlas 2021. [13-Nov-2023]. https://diabetesatlas.org Available. Accessed. - PubMed
-
- Centers for Disease Control and Prevention National diabetes statistics report. 2022. https://www.cdc.gov/diabetes/php/data-research/index.html Available.
-
- Plainsboro NJ. Ozempic. Novo Nordisk, Inc; 2025.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
