Collecting language, speech acoustics, and facial expression to predict psychosis and other clinical outcomes: strategies from the AMP® SCZ initiative
- PMID: 41093845
- PMCID: PMC12528486
- DOI: 10.1038/s41537-025-00669-z
Collecting language, speech acoustics, and facial expression to predict psychosis and other clinical outcomes: strategies from the AMP® SCZ initiative
Abstract
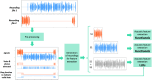
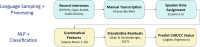
Speech-based detection of early psychosis is progressing at a rapid pace. Within this evolving field, the Accelerating Medicines Partnership® in Schizophrenia (AMP® SCZ) is uniquely positioned to deepen our understanding of how language and related behaviors reflect early psychosis. We begin with detailed standard operating procedures (SOPs) that govern every stage of collection. These SOPs specify how to elicit speech, capture facial expressions, and record acoustics in synchronized audio-video files-both on-site and through remote platforms. We then explain how we chose our sampling tasks, hardware, and software, and how we built streamlined pipelines for data acquisition, aggregation, and processing. Robust quality-assurance and quality-control (QA/QC) routines, along with standardized interviewer training and certification, ensure data integrity across sites. Using natural language processing parsers, large language models, and machine-learning classifiers, we analyzed Data Release 3.0 to uncover systematic grammatical markers of psychosis risk. Speakers at clinical high risk (CHR) produced more referential language but fewer adjectives, adverbs, and nouns than community controls (CC), a pattern that replicated across sampling tasks. Some effects were task-specific: CHR participants showed elevated use of complex syntactic embeddings in two elicitation conditions but not the third, underscoring the importance of the language sampling task. Together, these results demonstrate how computational linguistics can turn everyday speech into a scalable, objective biomarker, paving the way for earlier and more precise detection of psychosis.Video Link: https://vimeo.com/1112291965?fl=pl&fe=sh.
© 2025. The Author(s).
Conflict of interest statement
Competing Interests: The authors declare the following competing interests: K.A. is on the Australian Cognitive Impairment Associated with Schizophrenia Advisory Board for Boehringer Ingelheim and receives honorary funds. D.D. has received honorary funds for one educational seminar for CSL Sequiris. A.A. is a cofounder, serves as a member of the Board of Directors, as a scientific adviser, and holds equity in Manifest Technologies, Inc., and is a coinventor on the following patent: Anticevic A, Murray JD, and Ji JL: Systems and Methods for NeuroBehavioral Relationships in Dimensional Geometric Embedding, PCT International Application No. PCT/US2119/022110, filed Mar 13, 2019. E.C. has received speaker fees at non-promotional educational events. P.F.-P. has received research funds or personal fees from Lundbeck, Angelini, Menarini, Sunovion, Boehringer Ingelheim, Proxymm Science, Otsuka, outside the current study. J.K. has received speaking or consulting fees from Janssen, Boehringer Ingelheim, ROVI, and Lundbeck. C.M.D.-C. has received grant support from Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation, and honoraria or travel support from Angelini, Janssen, and Viatris; RU has received speaker fees at a non-promotional educational event: Otsuka: Consultancy for Viatris and Springer Healthcare. Honorary General Secretary, British Association for Psychopharmacology (unpaid). J.M.K. is a consultant to or receives honoraria and/or travel support and/or speakers bureau: Alkermes, Allergan, Boehringer-Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HealthRhythms, HLS Therapeutics, Indivior, Intracellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, Karuna Therapeutics/Bristol Meyer-Squibb, LB Pharmaceuticals, Mapi, Maplight, Merck, Minerva, Neurocrine, Newron, Novartis, NW PharmaTech, Otsuka, Roche, Saladax, Sunovion, Teva; RSK provides consulting to Alkermes, Boehringer-Ingelheim. S.W.W. has received speaking fees from the American Psychiatric Association and from Medscape Features. He has been granted a US patent no. 8492418 B2 for a method of treating prodromal schizophrenia with glycine agonizts. He owns stock in NW PharmaTech. C.A. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda; GDH has been a consultant for Bristol Myers Squibb. P.J.M. has been a consultant for Otsuka and TEVA; and Z.T. has been a consultant for Manifest Technologies. C.M.C. is an Associate Editor of Schizophrenia. All other authors report no competing interests.
Figures




References
-
- Andreasen N. C. Scale for the assessment of thought, language, and communication (TLC). Schizophr. Bull. 10.1093/schbul/12.3.473 (1986). - PubMed
-
- Andreasen, N. C. Thought, language, and communication disorders: II. Diagnostic significance. Arch. Gen. Psychiatry36, 1325–1330 (1979). - PubMed
-
- Andreasen, N. C. Thought, language, and communication disorders. I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch. Gen. Psychiatry.36, 1315–1321 (1979). - PubMed
-
- Andreasen, N. C. Scale for the assessment of negative symptoms (SANS). Br J Psychiatry.155, 53–58 (1989). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

