Research on the Synthesis and Conductivity of Titanium Oxycarbide
- PMID: 41095446
- PMCID: PMC12526258
- DOI: 10.3390/ma18194621
Research on the Synthesis and Conductivity of Titanium Oxycarbide
Abstract
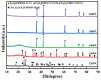
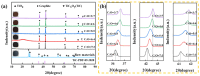
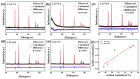
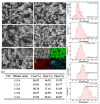
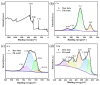
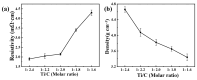
In this study, TiCxOy was produced by sintering in an argon atmosphere using carbon-thermal reduction with TiO2 and graphite powder as the initial materials. The sintered TiCxOy was analyzed using X-ray diffraction, scanning electron microscopy, and energy-dispersive X-ray spectroscopy. As the oxygen content increased, the grain color of the sintered TiCxOy gradually shifted from gray to reddish-brown. The structure of TiCxOy resembles that of a coral, with a uniform distribution of Ti, C, and O throughout the sample. Analysis using X-ray photoelectron spectroscopy reveals the presence of bivalent, trivalent, and tetravalent titanium. Utilizing General Structure Analysis System software (GSAS-II), the X-ray Diffraction data obtained were refined, revealing a gradual decrease in lattice parameters as the oxygen atom content increased. Furthermore, the conductivity and density of the single phase, determined through the four-probe method and the Archimedes method, respectively, exhibited an increase in tandem with the rise in C content.
Keywords: C/O molar ratio; TiCxOy; carbothermal reduction method.
Conflict of interest statement
Authors Shaolong Li, Tianzhu Mu and Fuxing Zhu were employed by the company Pangang Group Research Institute Co., Ltd. and author Shengwei Li was employed by the company Western Mining Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- He C., Zheng C., Dai W., Fujita T., Zhao J., Ma S., Li X., Wei Y., Yang J., Wei Z. Purification and Phase Evolution Mechanism of Titanium Oxycarbide (TiCxOy) Produced by the Thermal Reduction of Ilmenite. Minerals. 2021;11:104. doi: 10.3390/min11020104. - DOI
-
- Tian D., Wang M., Jiao H., Jiao S. Improved USTB Titanium Production with a Ti2CO Anode Formed by Casting. J. Electrochem. Soc. 2019;166:E226–E230. doi: 10.1149/2.0271908jes. - DOI
-
- Tian D., Wang M., Wang J., Tu J., Jiao S. Electrochemical Behaviors of Consumable Ti2CO@Al2O3 Anode for Ti Extraction by USTB Process. J. Electrochem. Soc. 2021;168:103508. doi: 10.1149/1945-7111/ac2d41. - DOI
-
- Jiao S., Zhu H. Electrolysis of Ti2CO solid solution prepared by TiC and TiO2. J. Alloy Compd. 2007;438:243–246. doi: 10.1016/j.jallcom.2006.08.016. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

