Transarterial Embolization for Refractory Non-Cervical-Origin Interscapular Pain Following Ultrasound-Guided Injection: A Retrospective Feasibility Study
- PMID: 41095715
- PMCID: PMC12523267
- DOI: 10.3390/diagnostics15192496
Transarterial Embolization for Refractory Non-Cervical-Origin Interscapular Pain Following Ultrasound-Guided Injection: A Retrospective Feasibility Study
Abstract
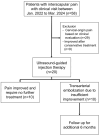
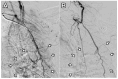
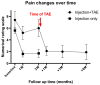
Objective: Chronic non-cervical-origin interscapular pain remains challenging to treat when refractory to conservative management and ultrasound-guided injections. This retrospective feasibility study aimed to assess the feasibility, procedural practicality, safety, and preliminary clinical outcomes of transarterial embolization (TAE) as a salvage therapy in this patient population. Methods: This single-center retrospective study included 20 patients with chronic interscapular pain (Numeric Rating Scale [NRS] score ≥5 for >3 months) who initially underwent ultrasound-guided injection therapy. Patients who experienced inadequate pain relief after 3 months (n = 10) proceeded to TAE, while the remaining 10 patients with sufficient relief formed the comparison group. TAE primarily targeted the transverse cervical artery using imipenem/cilastatin sodium as the embolic agent. Pain outcomes were assessed using NRS scores at 1, 3, and 6 months post-procedure. The primary outcome was pain reduction (≥50% decrease in NRS score), with secondary outcomes including technical success, medication use, and safety assessment. Results: The mean baseline NRS score for all patients was 6.5 ± 1.4, which decreased to 3.4 ± 2.0 at 1 month and 3.9 ± 2.5 at 3 months post-injection (p < 0.001). In the TAE group, the NRS score decreased from 7.4 ± 1.4 to 5.1 ± 1.1 at 1 month and 6.0 ± 1.4 at 3 months, indicating inadequate pain relief. In contrast, the injection-only group showed significant improvement, with NRS scores decreasing from 5.6 ± 0.5 to 1.6 ± 0.5 at 1 month and 1.7 ± 0.7 at 3 months (p < 0.001). The reduction in NRS scores was significantly less in the TAE group compared with the injection-only group (-2.2 vs. -4.0 and -28.7% vs. -71.4% at 1 month; -1.4 vs. -3.9 and -18.2% vs. -69.7% at 3 months; all p ≤ 0.001). Following TAE, the mean NRS score further decreased to 2.1 ± 0.7, 2.0 ± 1.1, and 1.9 ± 1.2 at 1, 3, and 6 months, respectively (p < 0.001), with clinical success rates of 90%, 100%, and 90% at these respective time points. At the final follow-up, the percentage of NRS score reduction was comparable between the TAE and injection-only groups (-74.8% vs. -69.7%, p = 0.397). No severe or life-threatening adverse events were observed; only self-limited adverse events were reported. Conclusions: In this retrospective feasibility study, TAE appeared safe and effective as a salvage therapy for patients with refractory non-cervical-origin interscapular pain unresponsive to injection therapy. Further prospective, randomized studies are needed to validate these findings, refine patient selection criteria, and optimize treatment outcomes.
Keywords: chronic musculoskeletal pain; imipenem/cilastatin sodium; interscapular pain; transarterial embolization; ultrasound-guided injection.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Young B.A., Sizer P.S., Day M. Thoracic facet dysfunction/Costotransverse joint pathology. In: Desai M., O’Brien J., editors. The Spine Handbook. Oxford University Press; Oxford, UK: 2018. pp. 209–230.
LinkOut - more resources
Full Text Sources

