Artificial Intelligence-Driven Multi-Omics Approaches in Glioblastoma
- PMID: 41096631
- PMCID: PMC12524854
- DOI: 10.3390/ijms26199362
Artificial Intelligence-Driven Multi-Omics Approaches in Glioblastoma
Abstract
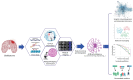
Glioblastoma (GBM) is the most common and aggressive primary brain tumor in adults. It is characterized by a high degree of heterogeneity, meaning that although these tumors may appear morphologically similar, they often exhibit distinct clinical outcomes. By associating specific molecular fingerprints with different clinical behaviors, high-throughput omics technologies (e.g., genomics, transcriptomics, and epigenomics) have significantly advanced our understanding of GBM, particularly of its extensive heterogeneity, by proposing a molecular classification for the implementation of precision medicine. However, due to the vast volume and complexity of data, the integrative analysis of omics data demands substantial computational power for processing, analyzing and interpreting GBM-related data. Artificial intelligence (AI), which mainly includes machine learning (ML) and deep learning (DL) computational approaches, now presents a unique opportunity to infer valuable biological insights from omics data and enhance the clinical management of GBM. In this review, we explored the potential of integrating multi-omics, imaging radiomics and clinical data with AI to uncover different aspects of GBM (molecular profiling, prognosis, and treatment) and improve its clinical management.
Keywords: artificial intelligence; deep learning; glioblastoma; machine learning; molecular classification; omics data; personalized medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

