Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age
- PMID: 41096774
- PMCID: PMC12525246
- DOI: 10.3390/ijms26199507
Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age
Abstract
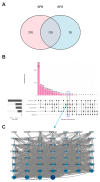
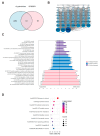
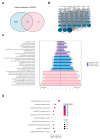
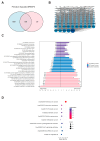
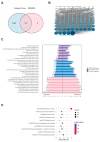
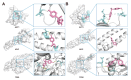
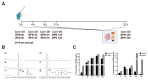
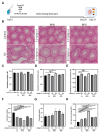
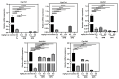
Accumulating evidence indicates that bisphenol A (BPA) analogs, including bisphenol B (BPB) and bisphenol S (BPS), disrupt testicular function and contribute to male reproductive dysfunction (MRD). However, whether BPA analogs are involved in MRD among middle-aged men remains inconclusive. Therefore, we selected cryptorchidism, erectile dysfunction, premature ejaculation, and testicular tumors as representative MRD conditions in middle-aged individuals, aiming to explore the molecular mechanisms that may be disrupted by bisphenols (BPs). By using GeneCards, STRING and Cytoscape, TP53, AKT1, and MYC were pinpointed as core targets associated with MRD. Enrichment analysis suggested that BPs may induce MRD by disrupting steroidogenesis. UPLC-MS/MS analysis showed that both BPB and BPS exhibit specific accumulation in the testes. Following 20-day exposure to 0.3 or 0.6 mg/kg body weight/day BPB or BPS, testosterone levels and the expression of hub genes were decreased. The molecular docking results demonstrated that both BPB and BPS can directly bind to members of the cytochrome P450 family, potentially interfering with sex hormone biosynthesis. Our study identified the targets and mechanisms through which BPB and BPS induce MRD in middle-aged males, thereby providing insights for the safety assessment of BPs.
Keywords: bisphenol B; bisphenol S; male reproduction dysfunction; middle-age; network toxicology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Fernández M.F., Arrebola J.P., Jiménez-Díaz I., Sáenz J.M., Molina-Molina J.M., Ballesteros O., Kortenkamp A., Olea N. Bisphenol A and Other Phenols in Human Placenta from Children with Cryptorchidism or Hypospadias. Reprod. Toxicol. 2016;59:89–95. doi: 10.1016/j.reprotox.2015.11.002. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

