The Biomarker Profile of Alzheimer's Disease for Disease-Modifying Treatment Eligibility: Questions and Debates
- PMID: 41096792
- PMCID: PMC12525501
- DOI: 10.3390/ijms26199531
The Biomarker Profile of Alzheimer's Disease for Disease-Modifying Treatment Eligibility: Questions and Debates
Abstract
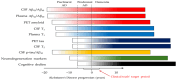
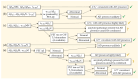
Alzheimer's disease (AD) is the most common cause of cognitive decline; currently, anti-amyloid monoclonal antibodies are available for clinical use as disease-modifying treatments, while many other substances are being tested in clinical trials. Molecular biomarkers for AD have been studied for more than two decades, and various guidelines and diagnostic recommendations have been published. However, there are still questions and controversies about the biomarker profile needed to confirm AD and the eligibility for such established treatments and clinical trials. Is amyloid positivity sufficient for eligibility, or is a biomarker for tau biochemistry/pathology also needed? What is the role of hybrid ratios combining amyloid and tau? Should we rely on plasma biomarkers alone? This review aimed to describe and discuss such questions and controversies.
Keywords: Alzheimer’s disease; amyloid beta; biomarkers; disease modifying treatments; donanemab; lecanemab; phospho-tau protein; tau protein.
Conflict of interest statement
None.
Figures
References
-
- World Health Organization Global Action Plan on the Public Health Response to Dementia 2017–2025. [(accessed on 6 July 2025)]. Available online: https://iris.who.int/handle/10665/259615.
-
- Koller E.J., Ibanez K.R., Vo Q., McFarland K.N., Gonzalez De La Cruz E., Zobel L., Williams T., Xu G., Ryu D., Patel P., et al. Combinatorial model of amyloid β and tau reveals synergy between amyloid deposits and tangle formation. Neuropathol. Appl. Neurobiol. 2022;48:e12779. doi: 10.1111/nan.12779. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

