Co-Targeting PD-1 and IL-33/ST2 Pathways for Enhanced Acquired Anti-Tumor Immunity in Breast Cancer
- PMID: 41096863
- PMCID: PMC12525228
- DOI: 10.3390/ijms26199600
Co-Targeting PD-1 and IL-33/ST2 Pathways for Enhanced Acquired Anti-Tumor Immunity in Breast Cancer
Abstract
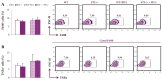
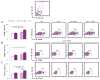
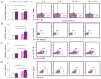
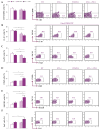
Despite advances in immunotherapy, the treatment of breast cancer still remains a major global problem. In a previous study, we showed that co-blockade of Interleukin-33/ST2 and Programmed death-1/Programmed death-ligand (PD-1/PD-L) signaling pathways strongly slows progression by enhancing the antitumor capacity of natural killer (NK) cells. The main aim of this study is to elucidate the exact effect of co-blockade on the T lymphocyte and macrophage effector cells. 4T1 cells were used to induct breast cancer in female BALB/C and BALB/C ST2-/- mice. The mice, both BALB/C and BALB/C ST2-/-, were treated with anti-PD-1 antibody on certain days. After the mice were sacrificed, T cells and macrophages were analyzed using flow cytometry; dual co-blockade increased significantly the percentage of M1 macrophages in the tumor microenvironment, followed by an increase in expression of CD86+ and TNFα+. T cell accumulation was significantly higher in the spleen and within the tumor microenvironment, with elevation in activation markers such as Interleukin-17, CD69, NKG2D, and FasL and a decrease in Interleukin-10 and FoxP3 expression. Co-blockade of the PD-1/PD-L axes and IL-33/ST2 axes shows promising results in reestablishing an effective immune response and offers a new perspective on improving immune response to breast carcinoma.
Keywords: IL-33; T cells; anti-PD-1; breast cancer; macrophages.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

