Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines
- PMID: 41096959
- PMCID: PMC12525465
- DOI: 10.3390/ijms26199694
Chemical Constituents from the Vietnamese Mangrove Avicennia marina: Two New Iridoid Glycosides and Their Cytotoxicity Against Cancer Cell Lines
Abstract
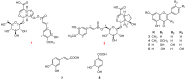
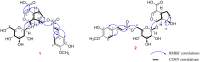
Avicennia marina, commonly known as the grey mangrove, is a salt-tolerant species widely distributed in coastal and estuarine ecosystems. Traditionally, it has been used in folk medicine to treat skin diseases, rheumatism, and ulcers due to its anti-inflammatory and antimicrobial properties. However, comprehensive studies on the chemical constituents and their pharmacological effects remain limited. The dried powder of the aerial parts of A. marina (3.6 kg) was successfully extracted three times with methanol (20 L × 3, each for 2 h) using a multifunctional ultrasonic cleaner operated at 25 °C with a 50% amplitude setting. In this study, the methanolic extract of the aerial parts of A. marina led to the isolation of eight compounds, including two previously unreported iridoid glycosides-avicenosides A and B (1 and 2)-and six known compounds: techtochrysin (3), 7,4'-di-O-methyl-apigenin (4), luteolin (5), kaempferol (6), trans-caffeic acid (7), and 3,4-dihydroxybenzoic acid (8). Their chemical structures were elucidated using nuclear magnetic resonance (NMR) spectroscopy and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) and compared with previously published data. Moreover, the absolute configuration of the sugar moieties in the new compounds was also identified. All isolated compounds were evaluated for their cytotoxicity against HepG2 and A549 cancer cell lines. The results indicate potential cytotoxicity of the secondary metabolites from A. marina and provide evidence of their promising role as lead compounds for the development of novel anticancer agents.
Keywords: Avicennia marina; avicenosides A and B; cytotoxicity; iridoid glycoside; mangrove plant; secondary metabolite.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Friess D.A., Rogers K., Lovelock C.E., Krauss K.W., Hamilton S.E., Lee S.Y., Lucas R., Primavera J., Rajkaran A., Shi S. The state of the world’s mangrove forests: Past, present, and future. Annu. Rev. Environ. Resour. 2019;44:89–115. doi: 10.1146/annurev-environ-101718-033302. - DOI
-
- Zhou P., Hu H., Wu X., Feng Z., Li X., Tavakoli S., Wu K., Deng L., Luo H. Botany, traditional uses, phytochemistry, pharmacological activities, and toxicity of the mangrove plant Avicennia marina: A comprehensive review. Phytochem. Rev. 2025:1–36. doi: 10.1007/s11101-025-10080-2. - DOI
-
- Warner R., Kaidonis M., Dun O., Rogers K., Shi Y., Nguyen T.T., Woodroffe C.D. Opportunities and challenges for mangrove carbon sequestration in the Mekong River Delta in Vietnam. Sustain. Sci. 2016;11:661–677. doi: 10.1007/s11625-016-0359-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

