Antidepressants Target the ST3GAL5-GM3 Lipid Pathway to Suppress Microglial Inflammation
- PMID: 41096998
- PMCID: PMC12524792
- DOI: 10.3390/ijms26199733
Antidepressants Target the ST3GAL5-GM3 Lipid Pathway to Suppress Microglial Inflammation
Abstract
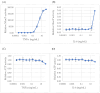
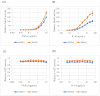
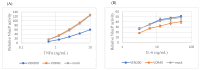
Major depression (MD) is associated with chronic inflammation and impaired neuroplasticity; however, the cellular mechanisms underlying antidepressant action remain incompletely understood. We performed transcriptomic profiling and functional validation in human microglia treated with venlafaxine (VEN) and vortioxetine (VOR), or with stable ST3GAL5 overexpression (ST3GAL5OE). Differential expression analysis, enrichment studies, and functional assays using NF-κB-RE-NlucP and SIE-NlucP reporter lines were conducted to assess the impact on inflammatory signaling. Microarray analysis identified 41 genes consistently upregulated and 316 consistently downregulated across VEN, VOR, and ST3GAL5OE conditions. Upregulated genes were enriched for synaptic organization, whereas downregulated genes were associated with nitric oxide biosynthesis and pro-inflammatory pathways, including Rap1, MAPK, and PI3K-Akt signaling. Functional assays confirmed that VEN and VOR suppressed cytokine-induced NF-κB and STAT3 activation, effects that were recapitulated by exogenous GM3 treatment and ST3GAL5 overexpression. Chronic exposure to VEN or VOR produced more modest, pathway-specific suppression, supporting convergence on the ST3GAL5-GM3 axis. These findings extend the conventional monoaminergic model of antidepressant action by highlighting the ST3GAL5-GM3 lipid remodeling axis as a novel regulatory pathway that attenuates microglial inflammatory signaling. Although validation in primary microglia and in vivo models is required, our results suggest that this axis could serve as both a therapeutic target and a candidate biomarker for inflammation-associated MD.
Keywords: GM3 ganglioside; ST3GAL5; antidepressants; cytokine signaling; inflammation; major depression; microglia; venlafaxine; vortioxetine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. - DOI - PMC - PubMed
-
- Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., Leucht S., Ruhe H.G., Turner E.H., Higgins J.P.T., et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. - DOI - PMC - PubMed
-
- Hirschfeld R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry. 2000;61((Suppl. S6)):4–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

