Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight
- PMID: 41097447
- PMCID: PMC12526424
- DOI: 10.3390/molecules30194026
Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight
Abstract
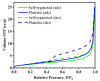
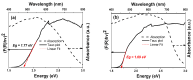
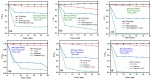
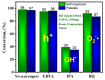
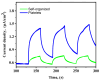
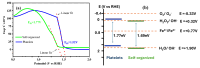
In this study, nanostructured ferberites (FeWO4) were synthesized via hydrothermal routes in an acidic medium. It was then investigated as an efficient photocatalyst for degrading organic dye molecules, with methylene blue (MB) as a model pollutant. The formation mechanism of ferberite revealed that the physical form of the precursor, FeSO4·7H2O, acts as a decisive factor in morphological evolution. Depending on whether it is in a solid or dilute solution form, two distinct nanostructures are produced: nanoplatelets and self-organized microspheres. Both structures are composed of stoichiometric FeWO4 (Fe: 49%, W: 51%) in a single monoclinic phase (space group P2/c:1) with high purity and crystallinity. The p-type semiconductor behavior was confirmed using Mott-Schottky model and the optical analysis, resulting in small band gap energies (≈1.7 eV) favoring visible absorption light. Photocatalytic tests under simulated solar irradiation revealed rapid and efficient degradation in less than 10 min under near-industrial conditions (pH 5). This was achieved using only a ferberite catalyst and a low concentration of H2O2 (4 mM) without additives, dopants, or artificial light sources. Advanced studies based on photocurrent measurements, trapping and stability tests were carried out to identify the main reactive species involved in the photocatalytic process and better understanding of photodegradation mechanisms. These results demonstrate the potential of nanostructured FeWO4 as a sustainable and effective photocatalyst for water purification applications.
Keywords: advanced water treatment; ferberite; hydrothermal synthesis; methylene blue degradation; nanostructured materials; photocatalysis; simulated sunlight irradiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- How Is Fast Fashion Degrading Our Water Resources? | Bosaq. [(accessed on 7 July 2025)]. Available online: https://bosaq.com/water-and-fashion-industry/
-
- Niinimäki K., Peters G., Dahlbo H., Perry P., Rissanen T., Gwilt A. The Environmental Price of Fast Fashion. Nat. Rev. Earth Environ. 2020;1:189–200. doi: 10.1038/s43017-020-0039-9. - DOI
-
- Bailey K., Basu A., Sharma S. The Environmental Impacts of Fast Fashion on Water Quality: A Systematic Review. Water. 2022;14:1073. doi: 10.3390/w14071073. - DOI
-
- Chavan R.B. Thirsty Textile and Fashion Industry PART I: Water Distribution on Earth and Virtual Water, Water Footprint Concepts. Latest Trends Text. Fash. Des. 2018;2:1–22. doi: 10.32474/LTTFD.2018.02.000150. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

