Real-World Outcomes and Biomarker Analysis Based on Routine Clinical, Laboratory, and Pathologic Parameters in Metastatic or Unresectable Esophageal Cancer Treated with First-Line Anti-PD-1 Plus Fluoropyrimidine and Platinum
- PMID: 41097677
- PMCID: PMC12524331
- DOI: 10.3390/cancers17193149
Real-World Outcomes and Biomarker Analysis Based on Routine Clinical, Laboratory, and Pathologic Parameters in Metastatic or Unresectable Esophageal Cancer Treated with First-Line Anti-PD-1 Plus Fluoropyrimidine and Platinum
Abstract
Background/objectives: The combination of anti-programmed death-1 (PD-1) inhibitors and chemotherapy is the standard first-line treatment for unresectable or metastatic esophageal squamous cell carcinoma (ESCC). However, real-world data remain limited, particularly regarding prognostic biomarkers.
Methods: This multi-institutional retrospective study analyzed patients with metastatic or unresectable ESCC who received first-line pembrolizumab or nivolumab plus fluoropyrimidine and platinum-based chemotherapy. Treatment regimens mirrored those in KEYNOTE-590 and CheckMate 648. Efficacy, safety, and prognostic factors were assessed. Prognostic factors were identified using multivariable Cox regression, and a point-based risk scoring system was developed.
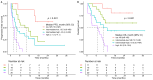
Results: Among 87 patients, the objective response rate was 48.3%, and the disease control rate was 77.0%. Median progression-free survival (PFS) was 5.6 months (95% CI, 4.5-8.7), and the median overall survival (OS) was 13.1 months (95% CI, 10.6-not reached). Grade 3-4 treatment-related adverse events occurred in 51.7% of patients. Eastern Cooperative Oncology Group (ECOG) performance status ≥ 2, elevated C-reactive protein, and lower programmed death-ligand 1 (PD-L1) combined positive score (CPS) were independently associated with worse PFS and OS. A prognostic risk score ranging from 0 to 5 based on these factors stratified patients into four prognostic groups with distinct survival outcomes. Median PFS ranged from not reached in the low-risk group to 2.1 months in the high-risk group. Stratifying PD-L1 CPS into three levels (<10, 10-49, ≥50) revealed a graded association between CPS and treatment outcomes, supporting the need for more nuanced PD-L1 evaluation beyond binary classification.
Conclusions: First-line anti-PD-1 therapy combined with chemotherapy demonstrated favorable real-world outcomes in ESCC. The proposed prognostic scoring system may help personalize treatment strategies.
Keywords: anti-PD-1 immunotherapy; esophageal squamous cell carcinoma; prognostic factors; real-world evidence; risk stratifications.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Morgan E., Soerjomataram I., Rumgay H., Coleman H.G., Thrift A.P., Vignat J., Laversanne M., Ferlay J., Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e642. doi: 10.1053/j.gastro.2022.05.054. - DOI - PubMed
-
- Ajani J.A., D’Amico T.A., Bentrem D.J., Cooke D., Corvera C., Das P., Enzinger P.C., Enzler T., Farjah F., Gerdes H., et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023;21:393–422. doi: 10.6004/jnccn.2023.0019. - DOI - PubMed
-
- Bleiberg H., Conroy T., Paillot B., Lacave A.J., Blijham G., Jacob J.H., Bedenne L., Namer M., De Besi P., Gay F., et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur. J. Cancer. 1997;33:1216–1220. doi: 10.1016/S0959-8049(97)00088-9. - DOI - PubMed
-
- Lee S.J., Kim S., Kim M., Lee J., Park Y.H., Im Y.H., Park S.H. Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: A randomized phase II study. BMC Cancer. 2015;15:693. doi: 10.1186/s12885-015-1716-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

