Spatial Transcriptomics Reveals Distinct Architectures but Shared Vulnerabilities in Primary and Metastatic Liver Tumors
- PMID: 41097737
- PMCID: PMC12523610
- DOI: 10.3390/cancers17193210
Spatial Transcriptomics Reveals Distinct Architectures but Shared Vulnerabilities in Primary and Metastatic Liver Tumors
Abstract
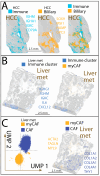
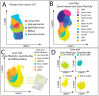
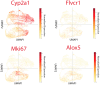
Background: Primary hepatocellular carcinoma (HCC) and liver metastases differ in origin, progression, and therapeutic response, yet a direct high-resolution spatial comparison of their tumor microenvironments (TMEs) within the liver has not previously been performed. Methods: We applied high-definition spatial transcriptomics to fresh-frozen specimens of one HCC and one liver metastasis (>16,000 genes per sample, >97% mapping rates) as a proof-of-principle two-specimen study, cross-validated in human proteomics and patients' survival datasets. Transcriptional clustering revealed spatially distinct compartments, rare cell states, and pathway alterations, which were further compared against an independent systemic dataset. Results: HCC displayed an ordered lineage architecture, with transformed hepatocyte-like tumor cells broadly dispersed across the tissue and more differentiated hepatocyte-derived cells restricted to localized zones. By contrast, liver metastases showed two sharply compartmentalized domains: an invasion zone, where proliferative stem-like tumor cells occupied TAM-rich boundaries adjacent to hypoxia-adapted tumor-core cells, and a plasticity zone, which formed a heterogeneous niche of cancer-testis antigen-positive germline-like cells. Across both tumor types, we detected a conserved metabolic program of "porphyrin overdrive," defined by reduced cytochrome P450 expression, enhanced oxidative phosphorylation gene expression, and upregulation of FLVCR1 and ALOX5, reflecting coordinated rewiring of heme and lipid metabolism. Conclusions: In this pilot study, HCC and liver metastases demonstrated fundamentally different spatial architectures, with metastases uniquely harboring a germline/neural-like plasticity hub. Despite these organizational contrasts, both tumor types converged on a shared program of metabolic rewiring, highlighting potential therapeutic targets that link local tumor niches to systemic host-tumor interactions.
Keywords: cancer metabolism; heme metabolism; plasticity; porphyrin metabolism; prostaglandin; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Arias I., Jakoby W., Popper M., Schachter D. The Liver-Biology and Pathobiology. Wiley-Blackwell; Hoboken, NJ, USA: 1988.
-
- Wu L., Yan J., Bai Y., Chen F., Zou X., Xu J., Huang A., Hou L., Zhong Y., Jing Z., et al. An invasive zone in human liver cancer identified by Stereo-seq promotes hepatocyte–tumor cell crosstalk, local immunosuppression and tumor progression. Cell Res. 2023;33:585–603. doi: 10.1038/s41422-023-00831-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

