A Comparison of Skeletal Muscle Diffusion Tensor Imaging Tractography Seeding Methods
- PMID: 41098059
- PMCID: PMC12529087
- DOI: 10.1002/nbm.70163
A Comparison of Skeletal Muscle Diffusion Tensor Imaging Tractography Seeding Methods
Abstract
The internal arrangement of a muscle's fibers with respect to its mechanical line of action (muscle architecture) is a major determinant of muscle function. Muscle architecture can be quantified using diffusion tensor magnetic resonance imaging-based tractography, which propagates streamlines from a set of seed points by integrating vectors that represent the direction of greatest water diffusion (and by inference, the local fiber orientation). Previous work in skeletal muscle has demonstrated that tractography outcomes are sensitive to the method for defining seed points, but this sensitivity has not been fully examined. To do so, we developed a realistic simulated muscle architecture and implemented three methods for tract seeding: seeding along the muscle-aponeurosis boundary with an updated procedure for rounding seed points prior to lookup in the muscle boundary mask and diffusion tensor matrices (APO); voxel-based seeding throughout the muscle volume at a uniform spatial frequency (VXL); and seeding near external and internal muscle boundaries (EDGE). We then implemented these methods in example human datasets. The updated aponeurosis seeding procedures allow more accurate and robust tract propagation from seed points. The voxel-based seeding methods had quantification outcomes that closely matched the updated aponeurosis seeding method. Further, the voxel-based methods can accelerate the overall workflow and may be beneficial in high throughput analysis of multi-muscle datasets. Continued evaluation of these methods in a wider range of muscle architectures is warranted.
Keywords: DTI; fiber‐tracking; freeware; muscle architecture; simulation; skeletal muscle.
© 2025 The Author(s). NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures


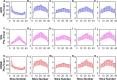
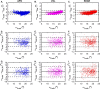

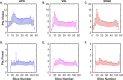
Update of
-
A Comparison of Skeletal Muscle Diffusion Tensor Imaging Tractography Seeding Methods.bioRxiv [Preprint]. 2024 Aug 30:2024.08.29.610343. doi: 10.1101/2024.08.29.610343. bioRxiv. 2024. Update in: NMR Biomed. 2025 Dec;38(12):e70163. doi: 10.1002/nbm.70163. PMID: 39257789 Free PMC article. Updated. Preprint.
References
-
- Sacks R. D. and Roy R. R., “Architecture of the Hind Limb Muscles of Cats: Functional Significance,” Journal of Morphology 173 (1982): 185–195. - PubMed
-
- Otten E., “Concepts and Models of Functional Architecture in Skeletal Muscle,” Exercise and Sport Sciences Reviews 16 (1988): 89–137. - PubMed
-
- Lieber R. L. and Friden J., “Functional and Clinical Significance of Skeletal Muscle Architecture,” Muscle & Nerve 23 (2000): 1647–1666. - PubMed
-
- Van Leeuwen J. L. and Spoor C. W., “Modelling the Pressure and Force Equilibrium in Unipennate Muscles With In‐Line Tendons,” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 342 (1993): 321–333. - PubMed
-
- Van Leeuwen J. L. and Spoor C. W., “Modelling Mechanically Stable Muscle Architectures,” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 336 (1992): 275–292. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

