Oil-water interfaces drive gold precipitation via microdroplet chemistry in thermal geological systems
- PMID: 41100660
- PMCID: PMC12557480
- DOI: 10.1073/pnas.2508673122
Oil-water interfaces drive gold precipitation via microdroplet chemistry in thermal geological systems
Abstract
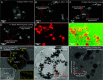
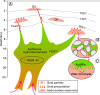
Sedimentary basins host high-grade gold mineralization at intersections of auriferous hydrothermal fluids and hydrocarbons. However, the precise mechanism of native gold formation associated with organic matter remains poorly understood. Here, we investigate gold precipitation at oil-water interfaces through in situ thermal experiments using various combinations of oil and HAuCl4-bearing solutions. Our results reveal that gold particles form spontaneously following the extensive generation and evolution of water microdroplets at oil-water interfaces at temperatures of 140 to 400 °C. We propose that electrons (e-), released from the conversion of hydroxide ions (OH-) to hydroxyl radicals (·OH) in water microdroplets, together with H atoms (·H) formed through electron transfer involving H3O+, and spontaneously generated H2O2 from·OH recombination, drive the reduction of Au3+ to Au0. The atomic gold progressively aggregates into Au0 clusters and Au nanoparticles (AuNPs), ultimately forming micrometer-scale gold particles and wires. This precipitation process occurs within minutes at temperatures above 350 °C and within hours below 200 °C. The experimentally produced gold particles exhibit textures like those in natural ore deposits. This interfacial microdroplet-induced mechanism provides a unique perspective on native gold formation in hydrocarbon-rich geosystems. Beyond its geological significance, this mechanism offers a potentially simplified approach for gold recovery from electronic waste without the need to introduce complex adsorbents or reducing agents into the waste stream.
Keywords: gold; microdroplet; oil–water.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Barnicoat A. C., et al. , Hydrothermal gold mineralization in the Witwatersrand basin. Nature 386, 820–824 (1997).
-
- Ding Z., et al. , Role of carbonaceous material in gold precipitation for orogenic gold deposits: A case study of the Bangbu gold deposit in southern Tibet, China. Ore Geol. Rev. 152, 105231 (2023).
-
- Fuchs S., Schumann D., Martin R. F., Couillard M., The extensive hydrocarbon-mediated fixation of hydrothermal gold in the Witwatersrand Basin, South Africa. Ore Geol. Rev. 138, 104313 (2021).
-
- Fuchs S. H. J., et al. , Gold and uranium concentration by interaction of immiscible fluids (hydrothermal and hydrocarbon) in the Carbon Leader Reef, Witwatersrand Supergroup, South Africa. Precambrian Res. 293, 39–55 (2017).
-
- Gaboury D., The neglected involvement of organic matter in forming large and rich hydrothermal orogenic gold deposits. Geosciences 11, 344 (2021).
Grants and funding
LinkOut - more resources
Full Text Sources

