1,1-polymerization of acetylene
- PMID: 41102165
- PMCID: PMC12533082
- DOI: 10.1038/s41467-025-64209-0
1,1-polymerization of acetylene
Abstract
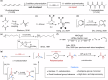
The polymerization of alkynes has long been a cornerstone of materials science, yielding a plethora of π-bond-rich materials and underpinning the 2000 Nobel Prize in Chemistry. While 1,2-polymerization of alkynes via 1,2-addition has been extensively studied, the 1,1-polymerization of carbon carbon triple bonds remains a formidable challenge due to the necessity of an alkyne rearrangement step during polymerization. Here we report the 1,1-polymerization of acetylene gas through a Cd-catalyzed iterative 1,1-carboboration process, achieving a broad terminal functionalization scope and functional group compatibility. This product, 1,1-polyacetylene (1,1-PA), also named as dendralene, exhibits unique physical and chemical properties, including an explosive tendency, distinguishing it from the 1,2-polyacetylene (1,2-PA) counterparts.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





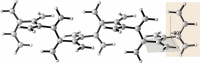
References
-
- Ito, T., Shirakawa, H. & Ikeda, S. Simultaneous polymerization and formation of polyacetylene film on the surface of concentrated soluble Ziegler-type catalyst solution. J. Polym. Sci., Part A Polym. Chem.34, 2533–2542 (1996).
-
- Shirakawa, H., Louis, E. J., MacDiarmid, A. G., Chiang, C. K. & Heeger, A. J. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun.1977, 578–580 (1977).
-
- Cao, Y. et al. Remarkable effects of anions on the chirality of thermoresponsive helical dendronized poly(phenylacetylene)s. Macromolecules54, 7621–7631 (2021).
-
- Mino, S. et al. Star-shaped polymers with helical polyacetylene arms. Comparison of solution- and solid-state properties with linear helical polyacetylenes. Macromolecules57, 10824–10834 (2024).
-
- Inoue, Y. et al. Platinum-mediated reversible cross-linking/decross-linking of polyacetylenes substituted with phosphine ligands: catalytic activity for hydrosilylation. Macromolecules55, 5711–5722 (2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

