Individualized prescriptive inference in ischaemic stroke
- PMID: 41102166
- PMCID: PMC12531334
- DOI: 10.1038/s41467-025-64593-7
Individualized prescriptive inference in ischaemic stroke
Abstract
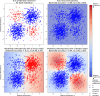
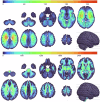
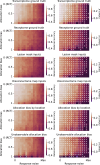
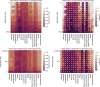
The gold standard in the treatment of ischaemic stroke is set by evidence from randomized controlled trials, typically using simple estimands of presumptively homogeneous populations. Yet the manifest complexity of the brain's functional, connective, and vascular architectures introduces heterogeneities that violate the underlying statistical premisses, potentially leading to substantial errors at both individual and population levels. The counterfactual nature of interventional inference renders quantifying the impact of this defect difficult. Here we conduct a comprehensive series of semi-synthetic, biologically plausible, virtual interventional trials across 100M+ distinct simulations. We generate empirically grounded virtual trial data from large-scale meta-analytic connective, functional, genetic expression, and receptor distribution data, with high-resolution maps of 4K+ acute ischaemic lesions. Within each trial, we estimate treatment effects using models varying in complexity, in the presence of increasingly confounded outcomes and noisy treatment responses. Individualized prescriptions inferred from simple models, fitted to unconfounded data, are less accurate than those from complex models, even when fitted to confounded data. Our results indicate that complex modelling with richly represented lesion data may substantively enhance individualized prescriptive inference in ischaemic stroke.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.G., J.C., S.O., G.R. and P.N. are affiliated with Hologen, a healthcare deep generative modelling company. C.F., G.P., J.K.R., T.X., H.R.J. and A.J. declare no competing interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

