External validation of PreOpNet to predict 30-day mortality after major non-cardiac surgery using digital electrocardiogram
- PMID: 41102258
- PMCID: PMC12533097
- DOI: 10.1038/s41746-025-01983-7
External validation of PreOpNet to predict 30-day mortality after major non-cardiac surgery using digital electrocardiogram
Abstract
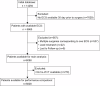
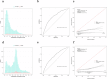
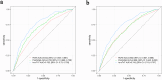
PreOpNet is a novel deep-learning algorithm using 12-lead digital electrocardiogram (ECG) for preoperative risk assessment of all-cause death and major adverse cardiac events (MACE) within 30 days. Its performance in European high-risk patients undergoing major non-cardiac surgery-the target population for guideline-recommended risk assessment-and comparison to high-sensitivity cardiac troponin T (hs-cTnT), is unknown. In a prospective European study (2014-2019), 6098 high-risk patients with available ECGs were enrolled. PreOpNet showed moderate discrimination for death (AUC 0.707) and MACE (0.675), but overestimated risk. It outperformed the revised cardiac risk index (RCRI) for death (AUC 0.644), but not for MACE (0.662). Hs-cTnT remained superior for both outcomes (AUC 0.762 and 0.743). Importantly, PreOpNet provided incremental prognostic value when combined with RCRI and/or hs-cTnT. PreOpNet has limited benefit for preoperative risk stratification in high-risk surgical patients as a stand-alone test. However, it holds promise when used in conjunction with RCRI and hs-cTnT. Clinical Trial Registration: ClinicalTrials.gov number: NCT02573532; https://www.clinicaltrials.gov/study/NCT02573532 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: P.L.A. has received research grants from the Swiss Heart Foundation (FF20079, FF21103 and FF24149) and speaker’s honoraria from Quidel, paid to the institution, Roche Diagnostics and Polymedco in the last 36 months, all outside the submitted work. J.B. is supported by an Edinburgh Doctoral College Scholarship and research grants from the University of Basel, the University Hospital of Basel, the Division of Internal Medicine, the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation, the Swiss National Science Foundation, the Swiss Heart Foundation, and has received honoraria from Siemens, Roche Diagnostics, Ortho Clinical Diagnostics, Quidel Corporation, and Beckman Coulter, and travel support from Medtron-ic and Vascularmedical, all outside the submitted work. C.M. reports receiving research support from the the Swiss National Science Foundation, the Swiss Heart Foundation, the University Hos-pital Basel, the University of Basel, Abbott, Astra Zeneca, Boehringer Ingelheim, Beckman Coul-ter, BRAHMS, Idorsia, Novartis, Ortho Clinical, Quidel, Roche, Siemens, SpinChip, Upstream, and Sphingotec, as well as speaker/consulting honoraria from Acon, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Idorsia, Osler, Novartis, Novo Nordisk, Roche, SpinChip, and Sanofi, all paid to the institution. F.M. has been supported by Deutsche Forschungsgemein-schaft (SFB TRR219, Project-ID 322900939) and Deutsche Herzstiftung. Saarland University has received scientific support from Ablative Solutions, Medtronic and ReCor Medical. Until May 2024, F.M. has received speaker honoraria/consulting fees from Ablative Solutions, Astra-Zeneca, Inari, Medtronic, Merck, Novartis, Philips and ReCor Medical. C.P. received research support from Roche Diagnostics and the Swiss Heart Foundation, as well as chaired an advisory board with honoraria from Roche Diagnostics paid to the institution. E.K. reports support from the Swiss Heart Foundation, the University Hospital Basel, Bangerter-Rhyner Foundation, and speaking/consulting fees from SpinChip, Boehringer Ingelheim. All other authors declare no competing interests.
Figures


References
-
- Nepogodiev, D. et al. Global burden of postoperative death. Lancet393, 401 (2019). - PubMed
-
- Lee, T. H. et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation100, 1043–1049 (1999). - PubMed
-
- Halvorsen, S. et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery: developed by the task force for cardiovascular assessment and management of patients undergoing non-cardiac surgery of the European Society of Cardiology (ESC) Endorsed by the European Society of Anaesthesiology and Intensive Care (ESAIC). Eur. Heart J.43, 3826–3924 (2022). - PubMed
-
- Biccard, B. M., Devereaux, P. J. & Rodseth, R. N. Cardiac biomarkers in the prediction of risk in the non-cardiac surgery setting. Anaesthesia69, 484–493 (2014). - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

