Characterizing the physiology of circulatory arrest in humans
- PMID: 41102468
- PMCID: PMC12532612
- DOI: 10.1038/s41591-025-03889-z
Characterizing the physiology of circulatory arrest in humans
Abstract
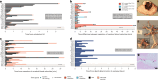
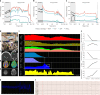
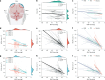
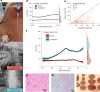
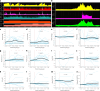
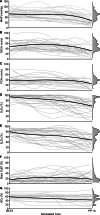
The dying process from circulatory arrest is an underexplored domain in humans and has transdisciplinary pertinence. Here we conducted a prospective, observational cohort study of the dying process in 39 adults, with a multimodal assessment of cerebrovascular and cardiovascular physiology. We found that cerebral blood velocities and brain tissue oxygen tensions ceased before systemic hemodynamics. The brain exhibited diffusion limitation of oxygen extraction during the dying process compared with extracranial tissues. Anterior and posterior brain circulations had differences in timing of cessation of circulation and physiologic responses during the dying process. Blood-based neurologic biomarkers from the brain did not change during the associated ischemia related to the dying process. Heart pathology was associated with the length of the dying process. This study provides proof-of-concept of an in vivo human model to comprehensively investigate severe cerebral ischemia and the human dying process. ClinicalTrials.gov registration: NCT06130033 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.D. is an unpaid volunteer for the Canadian Donation and Transplantation Research Program. M.S.S. is an unpaid volunteer for the Organ Donation and Transplantation Research Foundation of British Columbia since fall 2024. Both organizations provided pilot grant funding in April 2023 to supplement the research. While grant funding was provided, the funders had no role in the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. The remaining authors declare no competing interests.
Figures







References
-
- Guidelines for the determination of death: report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA246, 2184 (1981). - PubMed
-
- Greer, D. M. et al. Determination of brain death/death by neurologic criteria: the World Brain Death Project. JAMA324, 1078 (2020). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

