Lab-scale production of postbiotic proteins from Bifidobacterium adolescentis with antiviral and epithelial-protective properties
- PMID: 41103761
- PMCID: PMC12521213
- DOI: 10.3389/fmicb.2025.1646082
Lab-scale production of postbiotic proteins from Bifidobacterium adolescentis with antiviral and epithelial-protective properties
Abstract
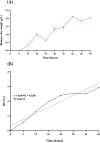
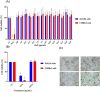
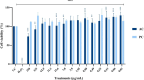
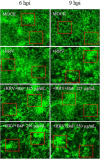
Postbiotics produced by probiotic bacteria are gaining attention as multifunctional, food-derived agents with potential applications in human and animal health. This study investigates the production and biological activity of protein-rich postbiotics from Bifidobacterium adolescentis, cultivated under controlled conditions in a 3-liter bioreactor as a laboratory-scale model for functional ingredient development. Culture parameters were improved, and one representative batch was selected for biological evaluation. The postbiotic preparation was tested for cytotoxicity using MA104 (renal) and C2BBe1 (intestinal) epithelial cell lines through viability and cell death assays, confirming its safety across a range of concentrations. To assess its functional activity, we evaluated its ability to reduce rotavirus infection and preserve epithelial integrity. The postbiotic significantly reduced viral infectivity and maintained cytoskeletal architecture in infected intestinal cells, supporting its potential protective role. These findings suggest that B. adolescentis-derived postbiotics may serve as safe and biologically active compounds with potential applications in intestinal health and viral infection management.
Keywords: Bifidobacterium adolescentis; antiviral activity; bioreactor; cytoskeletal integrity; functional ingredients; gut epithelium; postbiotics; rotavirus.
Copyright © 2025 Hernández, Quevedo, Cabrera and Ulloa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aguilar-Toalá J. E., Garcia-Varela R., Garcia H. S., Mata-Haro V., González-Córdova A. F., Vallejo-Cordoba B., et al. (2018). Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 75 105–114. 10.1016/j.tifs.2018.03.009 - DOI
-
- Armah G. E., Sow S. O., Breiman R. F., Dallas M. J., Tapia M. D., Feikin D. R., et al. (2010). Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet 376 606–614. 10.1016/S0140-6736(10)60889-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

