Ferulic acid mitigates 3-Nitropropionic acid-induced Huntington's disease via modulation of Nrf2/HO-1, TLR4/NF-κB, and SIRT1/p53 signaling pathways
- PMID: 41104344
- PMCID: PMC12521205
- DOI: 10.3389/fphar.2025.1678724
Ferulic acid mitigates 3-Nitropropionic acid-induced Huntington's disease via modulation of Nrf2/HO-1, TLR4/NF-κB, and SIRT1/p53 signaling pathways
Abstract
Background: Ferulic acid (FA) is a natural phenolic compound that has demonstrated effectiveness against Huntington's disease (HD). However, its exact mechanism remains unclear. Therefore, the current study aims to investigate FA's potential mechanism of action against 3-nitropropionic acid (3NP)-induced HD.
Methods: Adult male Wistar albino rats were administered FA orally (100 mg/kg) for 3 weeks, and 3NP (10 mg/kg) was intraperitoneally administered during the last 2 weeks to induce HD. Behavioral performance was assessed using the open field and hanging wire tests. Striatal tissue was analyzed using ELISA, qRT-PCR, Western blotting, histopathology, and immunohistochemistry.
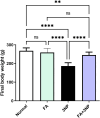
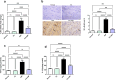
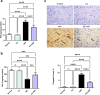
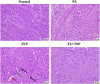
Results: Administration of 3NP led to weight loss, neurobehavioral deficits, oxidative damage, apoptotic cell death, and neuroinflammation. FA treatment mitigated these pathological changes by activating Nrf2/HO-1 signaling, a critical player in cellular redox balance. This beneficial effect was mirrored in restoring TAC levels and suppressing MDA. Moreover, FA suppressed TLR4/NF-κB inflammatory signaling, thereby reducing TNF-α and IL-1β levels. In addition, the anti-apoptotic properties of FA were confirmed by modulating SIRT1/p53 signaling, leading to Bcl-2 enhancement and caspase-3 downsizing. Furthermore, FA enhanced neuronal survival and plasticity confirmed by neurotrophic BDNF elevation. Histopathological and immunohistochemical analyses confirmed improved neuronal survival and reduced gliosis following FA treatment.
Conclusion: The current research demonstrates that FA exhibits potent neuroprotective effects in experimental HD by modifying Nrf2/HO-1, TLR4/NF-κB, and SIRT1/p53 signaling pathways. These findings provide new mechanistic insights into FA's potential role in managing HD.
Keywords: 3-Nitropropionic acid; Nrf2; SIRT1; TLR4; ferulic acid; neuroinflammation.
Copyright © 2025 Abdelgawad, Gendy, Zaghlool, Elesawy, Ragab, Kotb El-Sayed, El-Haddad, Mohamed, Alsalahat and Essa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Ahmed L. A., Darwish H. A., Abdelsalam R. M., Amin H. A. (2016). Role of rho kinase inhibition in the protective effect of fasudil and simvastatin against 3-nitropropionic acid-induced striatal neurodegeneration and mitochondrial dysfunction in rats. Mol. Neurobiol. 53, 3927–3938. 10.1007/s12035-015-9303-2 - DOI - PubMed
-
- Burnette W. N. (1981). “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112, 195–203. 10.1016/0003-2697(81)90281-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

