Update on Genetic Chorea
- PMID: 41104576
- PMCID: PMC12531716
- DOI: 10.1111/ene.70357
Update on Genetic Chorea
Abstract
Hereditary choreas are a clinically and genetically heterogeneous group of monogenic disorders in which chorea constitutes the core or an early-dominant feature. These conditions result from various genetic mutations affecting the structures and pathways involved in movement control, primarily the caudate and putamen, ultimately impairing the basal ganglia circuits involved in the regulation of movement, cognition, and behavior. This review focuses on the main forms of hereditary choreas, including Huntington's disease, neuroacanthocytosis syndromes, Huntington's disease phenocopies, benign hereditary chorea, and other less common genetic disorders presenting with chorea. We discuss the clinical, genetic, and pathophysiological features of each condition, alongside key aspects of phenomenology, examination, and complementary tests-including laboratory findings-to guide phenotype-driven genetic testing. We detail the characteristic features of key disorders while also highlighting less common but emerging conditions. This review aims to assist neurologists in recognizing and diagnosing hereditary choreas efficiently, including guidance on the selection of appropriate genetic tests, thereby reducing diagnostic delays, informing accurate counseling, and facilitating access to disease-specific interventions and clinical trials.
Keywords: Huntington disease‐like; Huntington's disease; benign hereditary chorea; hereditary chorea; neuroacanthocytosis.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
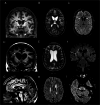
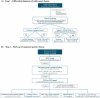
References
-
- Marsden C. D., Obeso J. A., and Rothwell J. C., “Clinical Neurophysiology of Muscle Jerks: Myoclonus, Chorea, and Tics,” Advances in Neurology 39 (1983): 865–881. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

