Electromagnetic Stimulation to Reduce Disability After Ischemic Stroke: The EMAGINE Randomized Clinical Trial
- PMID: 41105410
- PMCID: PMC12534854
- DOI: 10.1001/jamanetworkopen.2025.37880
Electromagnetic Stimulation to Reduce Disability After Ischemic Stroke: The EMAGINE Randomized Clinical Trial
Abstract
Importance: Ischemic stroke remains a leading cause of disability worldwide. Preliminary studies have suggested that noninvasive, frequency-tuned, low-intensity electromagnetic network targeting field (ENTF) stimulation may have recovery benefit for patients with stroke.
Objective: To evaluate the safety and effectiveness of ENTF therapy in reducing global disability among patients in the subacute ischemic stroke phase with moderate to severe disability and upper-extremity impairment.
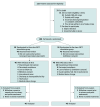
Design, setting, and participants: This multicenter, double-blind, sham-controlled, randomized clinical trial was conducted at 15 US-based acute care and inpatient rehabilitation facilities from December 2021 to November 2023. Participants were enrolled 4 to 21 days after a stroke and had a baseline modified Rankin Scale (mRS) score of 3 or 4 (moderate or moderately severe global disability) and Fugl-Meyer Assessment for Upper Extremity score of 10 to 45 (higher scores indicating better arm function). Target sample size was 150 participants. Participants were randomly allocated to receive either active or sham ENTF stimulation. Modified intention-to-treat approach was used in primary efficacy and safety analyses.
Intervention: Participants allocated to the active or sham ENTF stimulation were treated with a proprietary brain-computer interface-based stimulation device paired with an evidence-based, functional, repetitive, home-based physical and occupational exercise regimen for 45 one-hour sessions, 5 times per week within the first 90 days after a stroke.
Main outcomes and measures: The primary end point was change in global disability, assessed with the mRS (score range: 0 [indicating normal or no symptoms] to 6 [indicating death]), from baseline to day 90. Secondary end points were change from baseline to day 90 in upper-limb impairment, arm motor function, gait speed, hand function, and physical and functional limitations as well as day-90 health-related quality of life, each of which was assessed with a specific metric.
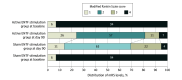
Results: The trial was stopped early after enrollment of 100 participants (50 in active group, 50 in sham group) when a promising zone threshold was not attained at planned interim analysis of the first 78 evaluable participants. Participants had a mean age of 59.0 (12.5) years and included 66 males (67.3%). The median (IQR) time from stroke to first ENTF treatment was 14 (12-19) days. Study groups were similar in age, sex, and baseline mRS scores, but imbalances were noted with participants in the active, compared with the sham, group having more right-hemisphere strokes (31 of 49 [63.3%] vs 22 of 49 [44.9%]), more severe upper-extremity impairment (Shoulder Abduction Finger Extension score <5; 31 of 49 [63.3%] vs 24 of 49 [49.0%]), and fewer small-vessel infarcts (14 of 49 [28.6%] vs 21 of 49 [42.9%]). For the primary outcome, the mean (SD) disability reduction on mRS at day 90 was not statistically significantly higher in the active group than in the sham group (-1.96 [0.12] vs -1.72 [0.12]), including mRS score of 0 to 1 attained in 12 participants (26.0%) vs 5 participants (10.0%) (odds ratio, 2.99; 95% CI, 0.96-9.30; P = .05). Point estimates for secondary outcomes favored the active group, although the differences were not statistically significant, in the prespecified analysis. No ENTF device-related serious adverse events were noted.
Conclusion and relevance: This trial found that ENTF therapy is safe. Although the difference between groups was not statistically significant, ENTF therapy may reduce global disability in patients with severe baseline disability after ischemic stroke. These results warrant confirmation in a higher powered pivotal trial of ENTF therapy.
Trial registration: ClinicalTrials.gov Identifier NCT05044507.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

