Suppression of pentraxin 3 inhibits human osteosarcoma cell metastasis by repressing MAZ through STAT3 pathway
- PMID: 41107857
- PMCID: PMC12535031
- DOI: 10.1186/s12935-025-04008-1
Suppression of pentraxin 3 inhibits human osteosarcoma cell metastasis by repressing MAZ through STAT3 pathway
Abstract
Background: Pentraxin 3 (PTX3), also known as TNF-inducible gene 14 protein (TSG-14), exerts pleiotropic roles in inflammation, immune responses, and a myriad of cancers, but there is limited knowledge regarding the role of PTX3 in the metastasis of human osteosarcoma.
Methods: Using RNA sequencing technology, transfection, western blotting, flow cytometry, and luciferase reporter, colony formation, quantitative reverse transcription–polymerase chain reaction (qRT-PCR), boyden chamber assays, and a xenograft model, the effects of PTX3 on cell migration and invasion in human osteosarcoma cells were investigated.
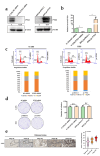
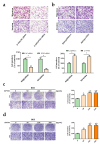
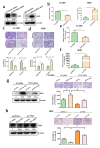
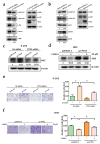
Results: PTX3 silencing repressed migration and invasion in U-2OS cells and PTX3 overexpression accelerated migration and invasion in HOS cells. Recombinant human PTX3 (rhPTX3) increased migration and invasion of HOS cells. In PTX3 knockdown U-2OS cells, the heatmap revealed the downregulation of Myc-associated zinc finger protein (MAZ), which is associated with the epithelial-mesenchymal transition pathway and osteosarcoma tissues. MAZ overexpression rescued the inhibition of migration by PTX3 knockdown in osteosarcoma cells. The activity of the MAZ promoter was reduced with PTX3 knockdown in osteosarcoma cells. While Stat3 overexpression rescued the inhibition of MAZ expression and migration by PTX3 knockdown in osteosarcoma cells. Moreover, in tail vein injection experiments, shPTX3 U-2OS cells reduced pulmonary metastasis formation in immunodeficient nude mice. By repressing MAZ through Stat3, suppression of PTX3 inhibits migration and invasion in osteosarcoma cells, and knockdown of PTX3 reduces pulmonary metastasis.
Conclusions: Our results raise interest in further study of PTX3’s anti-metastasis properties in osteosarcoma, providing molecular insight into PTX3’s role in osteosarcoma metastasis.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12935-025-04008-1.
Keywords: Invasion; MAZ; Migration; Osteosarcoma; Pentraxin 3.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Lu KH, Lu EW, Lin CW, Yang JS, Yang SF. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharmacol Ther. 2020;214:107611. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

