Perspective on alcohol-induced organ damage via autophagy-dependent cellular changes
- PMID: 41109131
- PMCID: PMC12554131
- DOI: 10.1016/j.redox.2025.103879
Perspective on alcohol-induced organ damage via autophagy-dependent cellular changes
Abstract
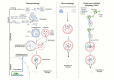
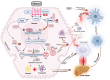
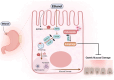
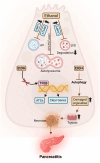
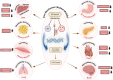
Alcohol-induced organ damage is a major global health concern and a leading cause of disability and mortality. In addition to the liver, chronic alcohol consumption adversely affects multiple organ systems, including the cardiovascular and neuromuscular systems, the gastrointestinal tract, adipose tissue, kidneys, pancreas, and the brain. Organ damage begins with necrosis (cell death) followed by tissue injury, which triggers immune responses aimed at tissue repair. However, with continued heavy drinking, these reparative responses transition into inflammatory responses which exacerbate tissue injury and accelerate disease progression. Macroautophagy, commonly referred to as autophagy, is a vital and evolutionarily-conserved digestive process in eukaryotic cells. While autophagy is well known for degrading obsolete proteins, and damaged organelles to prevent their accumulation, autophagy also plays critical roles in regulating innate and adaptive immunity and in modulating inflammation. This review describes evidence that chronic alcohol exposure and chronic alcohol consumption impair autophagy, contributing to dysfunction across multiple organ systems. We and others have explored the mechanisms by which alcohol disrupts autophagy to cause organ damage. This review aims to establish a cohesive understanding of these intracellular processes, which is essential for guiding future research toward targeting autophagy as a therapeutic strategy for alcohol-induced tissue injury. In summary, this comprehensive review integrates evidence from preclinical studies to present a unified perspective on how alcohol induces autophagy dysregulation and contributes to organ damage. It also highlights evidence-based recommendations from leading researchers in the field, offering valuable insights to advance autophagy-targeted therapies for mitigating the effects of alcohol-induced organ injury.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that the research was conducted with no commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Organization W.H. World Health Organization; 2018. Global Status Report on Alcohol and Health 2018.
-
- Hingson R.W., Zha W., White A.M. Drinking beyond the binge threshold: predictors, consequences, and changes in the US. Am. J. Prev. Med. 2017;52:717–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

