The microbiome in cancer
- PMID: 41112042
- PMCID: PMC12528007
- DOI: 10.1002/imt2.70070
The microbiome in cancer
Abstract
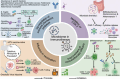
The human microbiome is now recognized as a central regulator of cancer biology, intricately shaping tumor development, immune dynamics, and therapeutic response. This comprehensive review delineates the multifaceted roles of bacteria, viruses, and fungi in modulating the tumor microenvironment and systemic immunity across diverse cancer types. We synthesize current evidence on how microbial dysbiosis promotes carcinogenesis via chronic inflammation, metabolic reprogramming, genotoxic stress, immune evasion, and epigenetic remodeling. This review emphasizes organ-specific microbiome signatures and highlights their potential as non-invasive biomarkers for early detection, treatment stratification, and prognosis. Furthermore, we explore the impact of intratumoral microbiota on cancer therapies, uncovering how microbial metabolites and host-microbe interactions shape therapeutic efficacy and resistance. Finally, advances in microbiome-targeted strategies, such as probiotics, fecal microbiota transplantation, and engineered microbes offer new avenues for adjunctive cancer therapy. This review provides a roadmap for future investigation and underscores the transformative promise of microbiome modulation in cancer prevention and treatment.
Keywords: cancer; microbiome; precision oncology; treatment; tumor microenvironment.
© 2025 The Author(s). iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Virtanen, Seppo , Saqib Schahzad, Kanerva Tinja, Ventin‐Holmberg Rebecka, Nieminen Pekka, Holster Tiina, Kalliala Ilkka, Salonen Anne. 2024. “Metagenome‐Validated Combined Amplicon Sequencing and Text Mining‐Based Annotations for Simultaneous Profiling of Bacteria and Fungi: Vaginal Microbiota and Mycobiota in Healthy Women.” Microbiome 12: 273. 10.1186/s40168-024-01993-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
