Shaping the future of probiotics, live biotherapeutic products, and fecal microbiota transplantation: 30 scientific recommendations from the CHINAGUT Conference
- PMID: 41112050
- PMCID: PMC12527994
- DOI: 10.1002/imt2.70083
Shaping the future of probiotics, live biotherapeutic products, and fecal microbiota transplantation: 30 scientific recommendations from the CHINAGUT Conference
Abstract
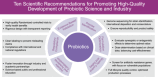
The 2025 CHINAGUT Conference has assembled a panel of 63 experts (30 scientists, 26 physicians, and 7 corporate R&D personnel) collaborated in three groups to present 30 scientific recommendations to advance probiotics, live biotherapeutic products, and fecal microbiota transplantation, addressing key issues on standardization, translation, supervision, regulation, and regulatory harmonization. These interdisciplinary guidelines aim to synthesize cutting-edge knowledge and practical needs to transform microbiota-based treatments from applications into precision-driven medical solutions, and serve as reference by scientific researchers, medical educators, pharmaceutical enterprises, clinicians, food and drug administrations, policymakers, and patients.
© 2025 The Author(s). iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
Canhui Lan is the founder and a shareholder of Beijing R‐Institute Co. Ltd., and receives a salary from the company. The company is dedicated to knowledge services in the life sciences and healthcare field, developing and operating the R‐base platform and its related products, and is an early funder of the journal iMeta. Canhui Lan also serves as one of the Executive Editors‐in‐Chief for iMeta, with responsibilities in operations and promotion. Additionally, he serves as a paid scientific consultant for several companies in the life sciences and healthcare industry. Xin Wang is the editor‐in‐chief of Beijing R‐Institute Co. Ltd., and receives a salary from the company. The company is dedicated to knowledge services in the life sciences and healthcare field, developing and operating the R‐base platform and its related products. Wanyin Tao is CTO and cofounder of Ibiome Inc. Faming Zhang conceived the concept of GenFMTer and transendoscopic enteral tubing and the devices (FMT Medical) related to them. Shuangjiang Liu is the Editor‐in‐Chief of iMeta, Jun Yu is the Advisory Board member of iMeta, Changtao Jiang and Jun Wang are the Associate Editors of iMeta, Heping Zhang and Fangqing Zhao are the Editorial Board members of iMeta. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication. The other authors declare no conflicts of interest.
Figures


References
-
- Metwaly, Amira , Kriaa Aicha, Hassani Zahra, Carraturo Federica, Druart Celine, Trebicka Jonel, Godoy Yolanda, et al. 2025. “A Consensus Statement on Establishing Causality, Therapeutic Applications and the Use of Preclinical Models in Microbiome Research.” Nature Reviews Gastroenterology & Hepatology 22: 343–356. 10.1038/s41575-025-01041-3 - DOI - PubMed
-
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis Koustantinos, Allende Ana, Alvarez‐Ordóñez Avelino, Bolton Declan, Bover‐Cid Sara, Chemaly Marianne, et al. 2023. “Update of the List of Qualified Presumption of Safety (QPS) Recommended Microbiological Agents Intentionally Added to Food or Feed as Notified to EFSA 18: Suitability of Taxonomic Units Notified to EFSA Until March 2023.” EFSA Journal 21: 8092. 10.2903/j.efsa.2023.8092 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
