Mitomycin C Induces Autophagy in Human Tracheal Fibroblasts and Suppresses Their Growth
- PMID: 41112678
- PMCID: PMC12529875
- DOI: 10.1002/lio2.70251
Mitomycin C Induces Autophagy in Human Tracheal Fibroblasts and Suppresses Their Growth
Abstract
Objective: Mitomycin C (MMC) is frequently used to prevent postoperative fibrosis in tracheal stenosis, yet its precise cellular mechanisms remain inadequately understood. This study aimed to elucidate the cytotoxic and autophagic effects of MMC on normal human tracheal fibroblasts (hTF) and human bronchial/tracheal epithelial cells (hTEC) to better understand its potential role in fibrosis regulation.
Methods: hTF and hTEC were exposed to MMC at concentrations of 0.01, 0.1, and 1 μg/mL for 24, 48, and 72 h. Cell proliferation, autophagy induction, and the expression of autophagy-related proteins were assessed using viability assays and Western blot analysis. Additionally, the effects of MMC on cell migration and fibroblast-to-myofibroblast transition were investigated.
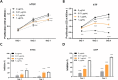
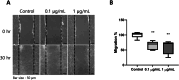
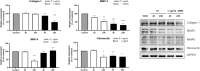
Results: MMC partially reduced hTEC proliferation without inducing autophagy. In contrast, MMC significantly suppressed hTF growth in a dose- and time-dependent manner while promoting autophagy. Western blot analysis revealed increased expression of LC3, ATG5, and Rab7 in MMC-treated hTF, along with reduced cyclin D1 levels. Furthermore, MMC attenuated TGFβ-induced αSMA expression in fibroblasts, suggesting an inhibitory effect on fibrosis-related cellular transformation.
Conclusion: These findings indicate that MMC suppresses human tracheal fibroblast proliferation through autophagy-mediated cell death while sparing epithelial cells. This dual effect underscores its potential as a targeted antifibrotic agent for tracheal stenosis management. Further research is needed to optimize MMC's application and elucidate its long-term impact on airway remodeling.
Level of evidence: 5.
Keywords: Mitomycin C; autophagy; bronchial/tracheal epithelial cell; tracheal fibroblast; tracheal stenosis.
© 2025 The Author(s). Laryngoscope Investigative Otolaryngology published by Wiley Periodicals LLC on behalf of The Triological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
