Quality of Measurement Properties in Patient Reported Outcomes Used in Adult Liver Transplant Candidates and Recipients: a Systematic Review
- PMID: 41113820
- PMCID: PMC12529315
- DOI: 10.3389/ti.2025.14497
Quality of Measurement Properties in Patient Reported Outcomes Used in Adult Liver Transplant Candidates and Recipients: a Systematic Review
Abstract
Objective: Patient Reported Outcome Measures (PROMs) are increasingly recognized in liver transplant (LT)-patients, yet recent evaluations of their quality are lacking. This systematic review gives a comprehensive overview of available PROMs in adults awaiting or undergoing LT and their measurement properties.
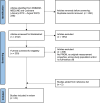
Method: A systematic search in MEDLINE, EMBASE, PubMed, and COCHRANE (01/2010-08/2023) included studies involving adult LT-candidates and/or recipients utilizing PROMs with original evaluations of measurement properties. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) was used to ascertain the quality of measurement properties.
Results: In total, 23 studies encompassing 35 PROMs were identified, including nine disease-specific and 26 generic PROMs. The (Short-form) Liver Disease Quality of Life ((SF-)LDQoL), Transplant Effects Questionnaire (TxEQ) and Post-Liver Transplant Quality of Life (pLTQ) were the most utilized disease-specific PROMs. Most studies demonstrated low-quality evidence for measurement properties. pLTQ demonstrated high-quality evidence for internal consistency, reliability, and responsiveness; the generic Hospital Anxiety and Depression Scale (HADS) showed strong evidence for internal consistency and construct validity.
Conclusion: Measurement properties in LT-patients remains of low-quality. pLTQ stands out for its superior methodological quality among disease-specific PROMs. For future studies, there is a strong recommendation to focus more on patients' subjective measures and their measurement properties.
Keywords: liver transplantation; measurement properties; patient reported outcome measures; quality of life; surgery.
Copyright © 2025 van Knippenberg, Powell-Brett, Joshi, Weeda and Hartog.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
-
- Annual Report on Liver Transplantation 2018/2019. NHS Blood and Transplant. (2018).
-
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses. (2020). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical