Modulation of functional network co-activation pattern dynamics following ketamine treatment in major depression
- PMID: 41113939
- PMCID: PMC12529346
- DOI: 10.1162/IMAG.a.936
Modulation of functional network co-activation pattern dynamics following ketamine treatment in major depression
Abstract
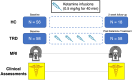
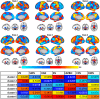
Ketamine produces fast-acting antidepressant effects in treatment-resistant depression (TRD). Prior studies have shown altered functional dynamics between brain networks in major depression. We thus sought to determine whether functional brain network dynamics are modulated by ketamine therapy in TRD. Participants with TRD (n = 58, mean age = 40.7 years, female = 48.3%) completed resting-state fMRI scans and clinical assessments (mood and rumination) at baseline and 24 h after receiving 4 ketamine infusions (0.5 mg/kg) over 2 weeks. Healthy controls (HC) (n = 56, mean age = 32.8 years, female = 57.1%) received the same assessments at baseline and after 2 weeks in a subsample without treatment. A co-activation pattern (CAP) analysis identified recurring patterns of brain activity across all subjects using k-means clustering. Statistical analyses compared CAP metrics including the fraction of time (FT) spent in a brain state, and the transition probability (TP) from one state to another over time and associations with clinical improvement. Follow-up analyses compared HC and TRD at baseline. Six brain state clusters were identified, including patterns resembling the salience (SN), central executive (CEN), visual (VN), default mode (DMN), and somatomotor (SMN) networks. Following ketamine treatment, TRD patients showed decreased FT for the VN (p = 7.4E-04) and increased FT for the CEN state (p = 1.9E-03). For TP metrics, SN-CEN increased (p = 5.8E-04) and SN-VN decreased (p = 3.6E-03). Decreased FT for the SN associated with improved rumination (p = 1.9E-03). At baseline, lower FT for CEN (p = 5.70E-04) and TP for SN-CEN (p = 0.016) and higher TP for SN-VN (p = 2.60E-03) distinguished TRD from HCs. CAP metrics remained stable over time in a subsample of HCs (n = 18). These findings suggest ketamine modulates brain network dynamics between SN, CEN, and VN in TRD, which may normalize dynamic patterns seen in TRD at baseline toward patterns seen in controls. Changes in SN state dynamics may correspond to improvements in ruminative symptoms following ketamine therapy.
Keywords: central executive network; co-activation patterns; dynamic functional connectivity; ketamine; rumination; salience network; treatment-resistant depression.
© 2025 The Authors. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
The authors have no competing financial interests or disclosures to make.
Figures


References
-
- Belleau, E. L., Bolton, T. A. W., Kaiser, R. H., Clegg, R., Cárdenas, E., Goer, F., Pechtel, P., Beltzer, M., Vitaliano, G., Olson, D. P., Teicher, M. H., & Pizzagalli, D. A. (2022). Resting state brain dynamics: Associations with childhood sexual abuse and major depressive disorder. NeuroImage. Clinical, 36, 103164. 10.1016/j.nicl.2022.103164 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
