Proteasomal deubiquitinating enzyme USP14/UCHL5 inhibitor bAP15 suppresses endoplasmic reticulum stress-mediated apoptosis and tumor growth in human chondrosarcoma
- PMID: 41113965
- PMCID: PMC12531285
- DOI: 10.62347/BCBI7081
Proteasomal deubiquitinating enzyme USP14/UCHL5 inhibitor bAP15 suppresses endoplasmic reticulum stress-mediated apoptosis and tumor growth in human chondrosarcoma
Abstract
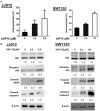
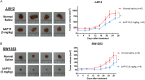
Chondrosarcoma is a malignant bone tumor with limited systemic options. Here, using the small-molecule probe bAP15 to inhibit proteasome-associated deubiquitinating enzymes (DUBs) USP14 and UCHL5, we evaluated the therapeutic concept of DUB inhibition in human chondrosarcoma. Immunohistochemistry showed higher USP14/UCHL5 expression in chondrosarcoma than in normal cartilage controls. In vitro, bAP15 increased Annexin V/PI-positive apoptosis and cleavage of caspase-3/PARP. Under our experimental conditions (low sub-micromolar exposure, 24-48 h), bAP15 led to a predominant accumulation of cells in G1, accompanied by p21 upregulation and reduced PCNA and phospho-histone H3. bAP15 was associated with lower phosphorylation of AKT/ERK and with the induction of ER-stress markers (GRP78, CHOP, IRE1α, caspase-4). In vivo, bAP15 suppressed xenograft growth. Collectively, these data support proteasome-associated DUB inhibition as a potential strategy in chondrosarcoma, with bAP15 serving as a chemical tool to probe this target class.
Keywords: Chondrosarcoma; bAP15; endoplasmic reticulum stress; proteasomal deubiquitinating enzyme.
AJCR Copyright © 2025.
Conflict of interest statement
None.
Figures




References
-
- Weinschenk RC, Wang WL, Lewis VO. Chondrosarcoma. J Am Acad Orthop Surg. 2021;29:553–562. - PubMed
-
- Chen JC, Huang C, Lee IN, Wu YP, Tang CH. Amphiregulin enhances cell migration and resistance to doxorubicin in chondrosarcoma cells through the MAPK pathway. Mol Carcinog. 2018;57:1816–1824. - PubMed
-
- MacDonald IJ, Lin CY, Kuo SJ, Su CM, Tang CH. An update on current and future treatment options for chondrosarcoma. Expert Rev Anticancer Ther. 2019;19:773–786. - PubMed
-
- Speetjens FM, de Jong Y, Gelderblom H, Bovee JV. Molecular oncogenesis of chondrosarcoma: impact for targeted treatment. Curr Opin Oncol. 2016;28:314–322. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
