Therapeutic challenges in HER2-targeted antibody therapies: trastuzumab and its ADC derivatives in breast cancer
- PMID: 41113985
- PMCID: PMC12531303
- DOI: 10.62347/FOWU3488
Therapeutic challenges in HER2-targeted antibody therapies: trastuzumab and its ADC derivatives in breast cancer
Abstract
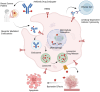
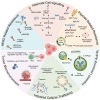
HER2 overexpression is associated with aggressive and poor patient outcome. HER2 has become a crucial target in cancer treatment, and the discovery of effective HER2-targeted therapies marked a significant milestone in treating HER2-positive cancers. This led to the approval of trastuzumab, the first HER2-targeted monoclonal antibody. Later, trastuzumab was used to develop antibody-drug conjugates (ADCs) for breast cancer, which have shown promising results. ADCs are combined trastuzumab with a cytotoxic drug to improve effectiveness while reducing side effects. Two ADCs, trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd), have been approved by the FDA for treating HER2-positive breast cancer. However, drug resistance has become a serious issue, reducing the long-term success of these treatments. This review explores key mechanisms of ADCs resistance including alteration in HER2 expression, antibody and payload-related resistance, altered cell signaling, impaired lysosomal and intracellular activity, and tumor microenvironment. By analyzing recent studies in ADCs resistance, this review provides an insight into ADC resistance mechanisms and potential strategies for improving therapeutic outcomes HER2 positive breast cancer.
Keywords: Antibody-drug conjugates (ADCs); T-DM1; T-DXd; breast cancer; drug resistance.
AJCR Copyright © 2025.
Conflict of interest statement
None.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Albagoush SA, Zubair M, Limaiem F. Tissue evaluation for HER2 tumor marker. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024.
-
- Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
