Adrenal insufficiency after megestrol acetate for fertility-sparing treatment of endometrial cancer
- PMID: 41114278
- PMCID: PMC12529359
- DOI: 10.1016/j.gore.2025.101961
Adrenal insufficiency after megestrol acetate for fertility-sparing treatment of endometrial cancer
Abstract
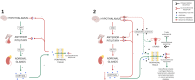
•AI is a rare yet potentially life-threatening side effect of MA when used for fertility sparing for EC/AH.•The presentation of AI is highly variable and clinicians should employ a low threshold for investigations.•AI can occur while on MA therapy or after abrupt withdrawal; MA should be tapered upon discontinuation.•Patients on MA may require stress dosing at times of surgery/procedures or acute illness.•Consider progestin IUD as a first line therapy, especially in patients with metabolic comorbidities.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Bulchandani D., et al. Megestrol acetate-associated adrenal insufficiency. Am. J. Geriatr. Pharmacother. 2008;6(3):167–172. - PubMed
-
- Delitala A.P., et al. Primary symptomatic adrenal insufficiency induced by megestrol acetate. Neth. J. Med. 2013;71(1):17–21. - PubMed
-
- Dev R., Del Fabbro E., Bruera E. Association between megestrol acetate treatment and symptomatic adrenal insufficiency with hypogonadism in male patients with cancer. Cancer. 2007;110(6):1173–1177. - PubMed
-
- Fried G., Stein M., Haim N. A rare event of megestrol acetate (Megace)-induced adrenal suppression in a breast cancer patient. Am. J. Clin. Oncol. 1997;20(6):628–629. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
