Medicare Part D Use and Costs for Immune-Mediated Neurologic Therapies
- PMID: 41114975
- PMCID: PMC12538365
- DOI: 10.1001/jamanetworkopen.2025.38277
Medicare Part D Use and Costs for Immune-Mediated Neurologic Therapies
Abstract
Importance: The expansion in the number of disease-modifying therapies (DMTs) for immune-mediated neurologic diseases raises critical questions about trends in their use and cost over time and whether this growth could be contributing to escalating financial burdens within the Medicare system.
Objective: To examine trends in claims and Medicare Part D payments for DMTs for immune-mediated neurologic diseases.
Design and setting: This retrospective economic evaluation analyzed Medicare Part D Prescriber Public Use Files from January 1, 2013, to December 31, 2022, restricting to prescription drug claims categorized under the clinician-defined neurology taxonomy. Only prescription drugs used as DMTs for multiple sclerosis, neuromyelitis optica spectrum disorder, and other immune-mediated neurologic disorders were included. To ensure accuracy, these medications were validated by 2 pharmacists. The data were analyzed between October 1, 2023, and November 12, 2024.
Main outcomes and measures: Ten-year trends in total payments, number of claims, and payment per claim for DMTs were assessed for their contribution to overall cost increases. Inflation adjustments were applied using both medical care-specific and prescription drug-specific indices.
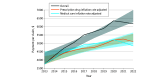
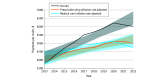
Results: Between 2013 and 2022, 63 unique drugs used as DMTs for multiple sclerosis, neuromyelitis optica spectrum disorder, and other immune-mediated neurologic disorders (eg, autoimmune encephalitis, myasthenia gravis) accounted for 6 526 278 claims and contributed $35 billion in Medicare drug expenditures. Between 2013 and 2022, annual claims for DMTs increased by 3.8% (from 549 766 to 570 298 claims). However, total payments for DMTs saw a disproportionate increase of 70.3% (95% CI, 13.5%-130.3%) from 2013 to 2022. After adjusting for medical care or prescription drug inflation, 2022 payments per claim increased by 29.1% (95% CI, 15.6% to 51.6%) and 35.9% (95% CI, 29.5%-50.3%), respectively, indicating that the rise in drug costs exceeded adjustments for medical care or prescription drug inflation rates. For the 29 DMTs available over the entire 10-year period, total claims decreased by 14.8% (from 546 026 to 465 089). Despite this reduction in claims, total payments increased by 60.5% (95% CI, 50.9%-90.6%). After adjusting for medical care inflation, the total payment increased significantly by 26.2% (95% CI, 10.5%-49.4%).
Conclusions and relevance: Over the past decade, Medicare Part D expenditures for DMTs targeting immune-mediated neurologic diseases have increased substantially, despite relatively stable claim volumes. These findings show that increases have consistently outpaced inflation rates, adding substantial economic pressure to federally funded neurologic care.
Conflict of interest statement
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

