Development of VEP-based biomarkers to assess plasticity states
- PMID: 41115871
- PMCID: PMC12537939
- DOI: 10.1038/s41398-025-03676-x
Development of VEP-based biomarkers to assess plasticity states
Abstract
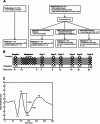
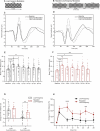
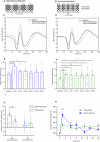
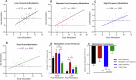
Disturbances in neuroplasticity are associated with many psychiatric and neurological disorders. Noninvasive electroencephalography (EEG) recordings of visually evoked potentials (VEPs) are promising for assessing plasticity in the human visual cortex, which may represent long-term potentiation (LTP). However, the variability in stimulation parameters limits the comparability and identification of optimal plasticity-inducing protocols. In this study, we systematically compared four VEP modulation protocols-low-frequency, repeated low-frequency, high-frequency, and theta-pulse stimulation-and assessed their effects on visual cortical plasticity. We analyzed 152 EEG recordings, where VEPs were evoked via a checkerboard reversal stimulus before and after low-frequency, repeated low-frequency, high-frequency, and theta-pulse stimulation. Changes in VEP amplitudes were measured from baseline to 2-28 min postmodulation. Low-frequency stimulation produced transient changes in plasticity, peaking at 2 min but dissipating within 12 min. Repeated low-frequency stimulation induced more sustained changes in plasticity, persisting for up to 22 min. High-frequency stimulation induced sharp but brief increases in plasticity indices, whereas theta-pulse stimulation was associated with moderate but prolonged changes in plasticity, lasting up to 28 min. These findings highlight the crucial influence of stimulation parameters on short- and long-term synaptic plasticity indices. Depending on the objective, a suitable induction protocol can be selected to optimize the desired effects, such as increasing sensitivity to drug effects or targeting longer-lasting plasticity outcomes. Optimized VEP paradigms have strong translational potential for assessing neuroplasticity deficits in individuals with psychiatric and neurodegenerative disorders, paving the way for the development of new biomarkers and therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval and informed consent: All procedures were performed in accordance with the Declaration of Helsinki and the relevant guidelines and regulations. The study was approved by the Ethics Committee of the Albert-Ludwigs-University Freiburg (approval numbers: 24-1237-S1 and 21-1113). Written informed consent was obtained from all participants prior to participation.
Figures

References
-
- von Bernhardi R, Bernhardi LE, Eugenín J. What is neural plasticity?. Adv Exp Med Biol. 2017;1015:1–15. - PubMed
-
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol. 2008;33:18–41. - PubMed
-
- Schulz PE, Fitzgibbons JC. Differing mechanisms of expression for short- and long-term potentiation. J Neurophysiol. 1997;78:321–34. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

