The microbial metabolite desaminotyrosine protects against graft-versus-host disease via mTORC1 and STING-dependent intestinal regeneration
- PMID: 41115965
- PMCID: PMC12537870
- DOI: 10.1038/s41467-025-65180-6
The microbial metabolite desaminotyrosine protects against graft-versus-host disease via mTORC1 and STING-dependent intestinal regeneration
Abstract
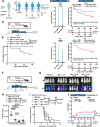
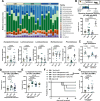
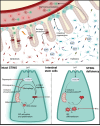
Changes in the intestinal microbiome and microbiota-derived metabolites predict clinical outcomes after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Here, we report that desaminotyrosine (DAT), a product of bacterial flavonoid metabolism, correlates with improved overall survival and reduced relapse rates in patients receiving allo-HSCT. In preclinical mouse models, treatment with synthetic DAT prevents graft-versus-host disease by protecting the intestinal barrier and promoting intestinal regeneration and contributes to graft-vs.-leukemia responses. DAT´s beneficial effects on intestinal regeneration remain effective despite broad-spectrum antibiotics-induced dysbiosis, also when administered by fecal microbiota transfer with flavonoid-degrading F. plautii. Mechanistically, DAT promotes mTORC1-dependent activation and proliferation of intestinal stem cells, with concomitant engagement of the innate immune receptor STING required to mitigate metabolic stress and maintain an undifferentiated stem cell state independently of type-I interferon responses. Additionally, DAT can skew T cells towards an effector phenotype to modulate graft-versus-leukemia responses. Our data uncover DAT's dual, tissue- and immune-modulating properties and underscore its potential in precision microbiome-based therapies to improve tissue regeneration and minimize immune-mediated side effects.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: HP: honoraria: Novartis, Gilead, Abbvie, BMS; Pfizer, Servier; Janssen-Cilag travel: Gilead, Janssen-Cilag, Novartis, Abbvie, Novartis; Jazz, Amgen Research: BMS. DW received research support from Novartis and honoraria from Sanofi, Incyte, Behring, Mallickrodt, Neovii and Takeda. F.B.: honoraria: BMS, Janssen, travel: Janssen. M.B-H.: honoraria: Pfizer Pharma GmbH; Sanofi Deutschland GmbH; travel: Novartis Pharma GmbH. S.H. is an employee of and hold equity interest in Roche/Genentech. WH: honoraria: Amgen, Novartis; travel: Janssen-Cilag, Amgen. E.T.O.: honoraria: BeiGene, AstraZeneca, travel: Janssen, Lilly. The content of this manuscript is part of a patent application of S.G., E.T.O. and H.P. All remaining authors declare no competing interest.
Figures





References
-
- O’Neill, A. T. & Chakraverty, R. Graft versus leukemia: current status and future perspectives. J. Clin. Oncol.39, 361–372 (2021). - PubMed
-
- Zeiser, R. et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med.382, 1800–1810 (2020). - PubMed
-
- Shouval, R. et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol.6, e573–e584 (2019). - PubMed
-
- Holler, E. et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant.20, 640–645 (2014). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

